- Research article
- Open Access
- Published:
Mutation of the cytosolic ribosomal protein-encodingRPS10Bgene affects shoot meristematic function in Arabidopsis
BMC Plant Biologyvolume12, Article number:160(2012)
Abstract
Background
Plant cytosolic ribosomal proteins are encoded by small gene families. Mutants affecting these genes are often viable, but show growth and developmental defects, suggesting incomplete functional redundancy within the families. Dormancy to growth transitions, such as the activation of axillary buds in the shoot, are characterised by co-ordinated upregulation of ribosomal protein genes.
Results
A recessive mutation inRPS10B,one of three Arabidopsis genes encoding the eukaryote-specific cytoplasmic ribosomal protein S10e, was found to suppress the excessive shoot branching mutantmax2-1.rps10b-1mildly affects the formation and separation of shoot lateral organs, including the shoot axillary meristems. Axillary meristem defects are enhanced whenrps10b-1is combined with mutations inREVOLUTA,AUXIN-RESISTANT1,PINOIDor another suppressor ofmax2-1,FAR-RED ELONGATED HYPOCOTYL3. In some of these double mutants, the maintenance of the primary shoot meristem is also affected. In contrast, mutation ofALTERED MERISTEM PROGRAMME1suppresses therps10b-1axillary shoot defect. Defects in both axillary shoot formation and organ separation were enhanced by combiningrps10b-1withcuc3,a mutation affecting one of three Arabidopsis NAC transcription factor genes with partially redundant roles in these processes. To assess the effect ofrps10b-1on bud activation independently from bud formation, axillary bud outgrowth on excised cauline nodes was analysed. The outgrowth rate of untreated buds was reduced only slightly byrps10b-1in both wild-type andmax2-1backgrounds. However,rps10b-1strongly suppressed the auxin resistant outgrowth ofmax2-1buds. A developmental phenotype ofrps10b-1, reduced stamen number, was complemented by the cDNA of another family member,RPS10C, under theRPS10Bpromoter.
Conclusions
RPS10Bpromotes shoot branching mainly by promoting axillary shoot development. It contributes to organ boundary formation and leaf polarity, and sustainsmax2-1bud outgrowth in the presence of auxin. These processes require the auxin response machinery and precise spatial distribution of auxin. The correct dosage of protein(s) involved in auxin-mediated patterning may beRPS10B-dependent. Inability of otherRPS10gene family members to maintain fully S10e levels might cause therps10b-1phenotype, as we found no evidence for unique functional specialisation of eitherRPS10Bpromoter or RPS10B protein.
Background
Shoot branching exemplifies two characteristic aspects of plant development. First, the body plan is generated by the production of repetitive modules. Second, the timing of the initiation, subsequent growth, and the final morphology of these modules are flexible and responsive to internal and external cues. This second aspect suggests that plants possess mechanisms to modulate their cellular growth machinery, including complex and energy-demanding processes such as ribosomal biogenesis, cell divison and cell expansion.
During post-embryonic growth of the shoot, secondary shoot meristems can generate new growth axes. These secondary meristems include leaf-associated, branch-forming axillary meristems, and reproductive, floral meristems [1].In many respects, these secondary meristems resemble the primary shoot meristem, which gives rise to the primary shoot axis. A common set of regulatory genes acts in their formation and patterning [2].Few genes, such as the ArabidopsisRAXfamily [3,4] seem to function exclusively in the formation of secondary shoot meristems, possibly as position specific initiators of the shoot meristematic programme. Some of the common functions are encoded by small gene families whose members vary in their contribution with respect to meristem position, such that mutation of one family member results in a secondary shoot meristem-specific phenotype. For example.in Arabidopsis,loss ofREVOLUTA (REV), one of a family of five class IIIHOMEODOMAIN LEUCINE ZIPPER (HDZIPIII)transcription factor genes, leads to partial loss of axillary meristems and causes premature arrest of some floral meristems [5,6].However, if two other family members,PHAVOLUTAandPHABULOSA, are mutated in addition toREV, the embryonic shoot meristem fails to form [7,8].Similarly, within the three-memberCUP-SHAPED COTYLEDON(CUC) gene family,CUC2andCUC3overlap in axillary meristem formation, while all three genes contribute to the formation of the primary shoot meristem [9- - - - - -12].
Secondary shoot meristems initiate in zones whereCUCandHDZIPIIIexpression overlap [2].PostembryonicCUCexpression strongly marks the boundaries of initiating lateral organs and has also been detected, at a low level, at the meristem centre [10,11,13,14].CUC3for example, marks the adaxial boundary of developing leaf primordia, where secondary meristems will form [12].HDZIPIIIexpression is initially continuous, spanning the meristem centre and the adaxial half of initiating leaves, but the leaf domain separates with its displacement from the growing meristem summit [6,15].The abaxial side of organ primordia is marked by expression of genes from the four-memberKANADI (KAN)family. These may limit shoot meristematic activity, because ectopicKANexpression abolishes shoot meristem formation, and multiple loss-of functionkanseedlings form ectopic lateral organs [16- - - - - -19].而这些和其他一些转录factor genes are clearly involved in establishing and patterning shoot meristems, it is less clear whether and how they affect the rate of meristematic growth and organ production. For example,HDZIPIIIfamily members appear to regulate the size of the central stem-cell containing zone in shoot meristems [8,20- - - - - -22], and this might affect meristem activity.CUCexpression marks zones of reduced growth within the shoot meristem [23], but also in other tissues [24].
Many of the axillary shoot meristems initiated during the lifetime of a plant cease growing after a short period, forming a small dormant bud in the leaf axil. Due to their ability to resume growth rapidly in response to an activating signal, axillary buds have been used as a model to study the regulation of meristematic activity in plants. Subtractive gene cloning in pea, and microarray analysis in Arabidopsis, show that bud activation involves a rapid, strong and coordinate upregulation of cell-cycle and protein synthesis-related genes, including many ribosomal protein (r-protein) genes, which precedes the onset of growth [25,26].Analysis of the promoter motifs shared by these genes points to possible control by members of the TCP (TEOSINTE BRANCHED / CYCLODEA / PROLIFERATING CELL FACTORS 1 and 2) transcription factor family [26].Of the two types of TCPs, class I is associated with growth activation and class II with growth arrest; and the DNA binding motifs identified for each class overlap partially, raising the possibility of competitive regulation via shared promoter elements [27].In support of a role of TCPs in axillary bud growth control, loss of function of axillary shoot-meristem-specific class II TCPs, such as theBRANCHED1 (BRC1)andBRC2genes of Arabidopsis, is associated with constitutive bud activation [28,29].The correlation between the expression of such bud-specific class IITCPgenes and the extent of bud growth repression is generally good, but not absolute [30].One possible explanation for this is the involvement of co-regulators of bud growth such as positively-acting TCPs.
The plant hormone auxin plays a dual role in shoot meristem growth, acting both locally along with patterning genes within the meristem, and as a long-distance signal to coordinate meristem activities within the shoot. Its patterning role has been clarified in the last decade. Transient local auxin maxima form and induce lateral organ formation in the peripheral zone of shoot meristems. These are created through directional auxin transport involving PIN1 and possibly other members of the PIN-formed protein family [31].The protein kinase PINOID [32] is required for the observed dynamic directional changes in PIN plasma membrane localisation and auxin transport direction [33,34].Organogenesis is thought to be induced via auxin-receptor mediated activation of members of the AUXIN RESPONSE FACTOR (ARF) transcription factor family [35], several of which are expressed at the shoot apex [36].These might, directly or indirectly, modulate the expression of meristem patterning genes. For example, auxin-mediated repression is thought to restrictCUCexpression to the boundaries of initiating organs [37].In contrast, someHD-ZIPIIIfamily members are auxin-induced [38].
Lateral organ development is accompanied by an inward movement of auxin through the centre of the organ primordium towards the vasculature in the subtending shoot axis [39,40].It is thought that this triggers vascular differentiation in an interplay with the adaxialHDZIPIII, abaxialKAN, andARFgenes expressed within this zone [41,42], and establishes continuity with the pre-existing vasculature, in which auxin moves in a strictly basipetal (shoot-to-root) direction in the xylem parenchyma. Interestingly auxin moving in this polar transport stream (PATS) in the shoot axis has long been known to inhibit axillary shoot meristem activity in an indirect manner. These observations have been integrated into a model where both apical and axillary shoot meristem activities are governed by the ability to canalize auxin transport from developing organ primordia into pre-existing vasculature [43- - - - - -45].In addition, auxin in the PATS seems to control the production of other signals, which move root-to-shootwards in the xylem and might enter axillary shoots and regulate their growth. Auxin suppresses the biosynthesis of cytokinins [46,47], which can promote the growth of axillary buds when directly applied to them [48], and promotes the biosynthesis of the recently-discovered strigolactones [49- - - - - -52], which can inhibit axillary buds upon direct application [53].
more axillary growth2-1(max2-1) is a strigolactone signalling mutant which shows constitutive axillary bud activation [54- - - - - -56].In a screen for second-sitemax2-1branching suppressors, we unexpectedly identified a mutation inRPS10B, one of three genes encoding protein S10e of the cytoplasmic ribosome, whose role in supporting shoot meristematic function we describe here.
Results
A recessive mutation in cytosolic ribosomal protein RPS10B partially suppressesmax2-1
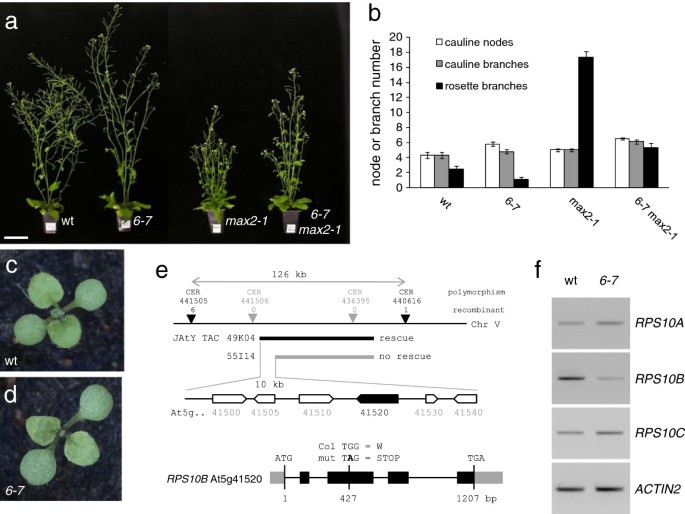
The strigolactone-insensitivemax2-1mutant produces an excessive number of inflorescence branches from rosette leaf axils [54].To identify novel regulators of shoot branching, we performed a suppressor screen in this genetic background. In one of the isolates,6-7, a recessive, second-site mutation, significantly reduced rosette branching. In addition,6-7shoots were slightly taller thanmax2-1and their primary inflorescences had a slightly higher number of cauline, leaf-bearing nodes (Figure 1a, b). We temporarily named the suppressor mutation in this line67. After backcrossing6-7to wild-type Columbia, these traits were also detected in the wild-typeMAX2background, although the effect on branching was less striking, and could not be readily used to map the suppressor. A pointed juvenile leaf phenotype that co-segregated with the branching habit was instead used (Figure 1c, d).67was crossed to Landsberg-erecta, and the locus was mapped to a 126 kb region on chromosome 5 by assessing co-segregation of DNA polymorphisms between Landsberg and Columbia in mutant individuals from the F2of this cross. JAtY TAC library clones in pYLTAC17 [57] containing large wild-type genomic inserts from the mapping interval were transformed into the mutant and assessed for rescue. This defined six candidate genes, whose coding regions were amplified from6-7and sequenced (Figure 1e). The sole divergence from wild type was a G to A transition, which introduced a premature termination codon in At5g41520 (RPS10B), one of three Arabidopsis genes encoding cytoplasmic ribosomal protein S10e.RPS10Btranscript level was lower in6-7than in the wild type (Figure 1f), suggesting nonsense-mediated decay. Identity ofRPS10Bas the suppressor gene was confirmed by mutant rescue with a wild-typeRPS10Bgenomic construct (Additional file1: Table S1), and the mutant allele was namedrps10b-1.
6-7, a partial suppressor ofmax2-1,affects ribosomal protein geneRPS10B.(a,b) Effect of6-7on shoot architecture and branching in the wild-typeMAX2and in themax2-1mutant background. (a) Plants aged 6 weeks. Scale bar: 5 cm. (b) Number of cauline nodes, cauline branches and rosette branches (≥0.5 cm) at maturity (Average ± SEM, n = 10). (c,d) The first leaves of6-7mutant seedlings (d) are slightly more pointed than those of wild-type (c) seedlings. (e)6-7carries a mutation inRPS10B, one of three Arabidopsis genes encoding ribosomal protein S10e. Gene mapping to a 126 kb interval on chromosome 5. Population size: about 1600 mutant individuals. Mutant rescue by JAtY TAC clone 49 K04, but not by 55I14, defined six candidate genes. Only one of these,RPS10B, carried a nonsense mutation in its coding region. (f) RT-PCR analysis showing reducedRPS10Btranscript levels in6-7. The primers used for RT-PCR are given in Table 5.
rps10b-1affects axillary shoot initiation and growth
With wild-type Columbia plants grown in long photoperiods, floral transition is the trigger for axillary shoot initiation. The axillary shoots activate to form inflorescence branches in an apical-basal wave, i.e. from the cauline leaf axils, situated along the primary inflorescence, towards the rosette leaf axils [58].In the wild type, only a few of the topmost rosette leaf axils produce branches, while more basal rosette axils carry arrested buds. Inmax2-1, neither the timing of axillary shoot initiation nor the outgrowth sequence is altered, but nearly all the rosette axils produce a branch [54].
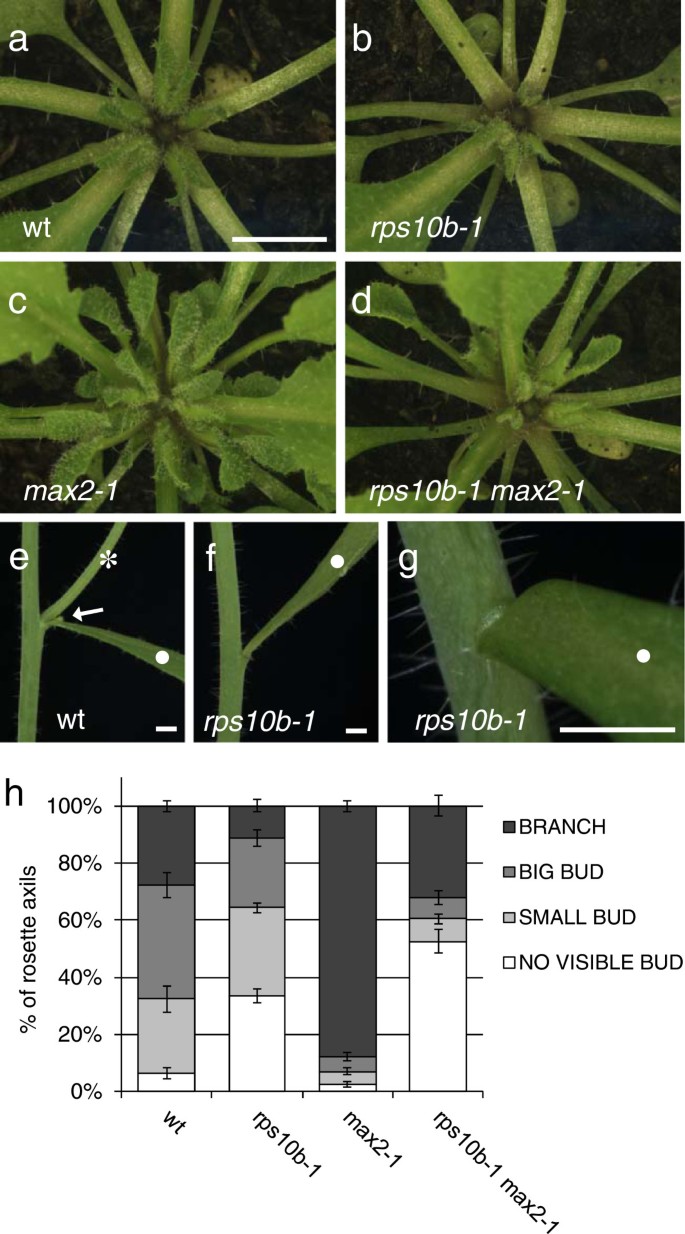
Therps10b-1mutation caused a reduction in axillary shoot size at equivalent nodal positions in the rosettes of bothMAX2andmax2-1plants (Figure 2a–d). In addition, one or two axils at the top of the rosette often appeared to be empty. A small proportion of therps10b-1茎生叶的腋我们re also empty (Figure 2e–g, Table 1), and remained so until maturity. This indicates thatrps10b-1affects axillary shoot initiation. Either a delay in axillary shoot formation, or an additional effect on axillary bud growth rate, might cause the reduced size ofrps10b-1buds.
rps10b-1affects axillary bud initiation and growth in the wild-type and themax2-1mutant backgrounds.(a-d) Rosette centres seen from above at early flowering stage. The primary inflorescences were between 1.7 and 2 cm long and were removed to reveal the rosette leaf axils. Scale bar in (a) for (a-d) 5 mm. (e-g)rps10b-1affects axillary shoot formation at cauline nodes. Scale bars in (e-g) 2 mm. (e) Wild-type cauline node with a leaf (white dot), a cauline branch (white star) and a small accessory axillary bud (white arrow). (f,g) Some cauline leaf axils ofrps10b-1appear empty. (h) Quantitative analysis of rosette axillary shoot development at the reproductive stage (when the tenth flower on the primary inflorescence opened). For ten rosettes per genotype, all the leaf axils were examined under a dissecting microscope and the developmental stage of the axillary shoots scored into four classes given in the key (defined in detail in the Results section). The percentages of rosette nodes occupied by each class were calculated for each individual plant and the average percentages (±SEM) for each genotype are shown.
To quantify these phenotypes, we examined flowering plants under a dissecting microscope and assessed axillary shoot development at consecutive nodal positions throughout the rosette. Four developmental stages were defined, and the proportions of rosette axils at each stage were calculated for ten individual plants per genotype (average proportions ± SEM shown in Figure 2h). The stages were defined as follows: 1. Branches (inflorescence length above 3 mm), 2. Big axillary buds whose inflorescence had not yet significantly elongated. 3. Small buds with leaf primordia clearly visible but shorter than 2 mm. 4. Apparently empty axils lacking visible axillary leaf primordia (it was not possible at the magnification used to determine whether an axillary meristem had been initiated or not). The frequency of class 4 was negligible in both wild-type andmax2-1rosettes, but these genotypes differed with respect to the proportions of the three more advanced classes. Compared to the wild type,max2-1showed a dramatic increase in the most advanced class, balanced by a decrease of the two intermediate classes. In contrast, forrps10b-1in both theMAX2andmax2-1backgrounds, the proportion occupied by the most advanced class decreased, and this was balanced by an increase in the proportion of apparently empty axils, with little change in the intermediate classes. These results indicate thatRPS10Bpromotes axillary shoot development from an early stage, including both axillary bud formation and possibly subsequent bud growth. In contrast,MAX2represses only the later stages of bud activity [54], suggesting thatRPS10Bacts at least in part independently ofMAX2.
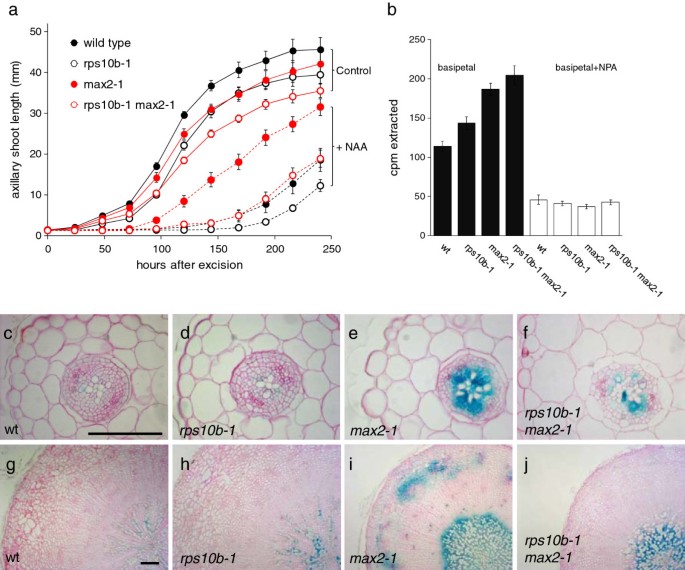
To assess whetherrps10b-1影响腋窝射杀增长独立于我nitiation, we studied the outgrowth kinetics of axillary inflorescences on isolated cauline nodes. Nodal explants, consisting of a cauline axillary bud smaller than 2 mm and 5–7 mm of the primary inflorescence stem above and below the node, were inserted between two agar slabs in a Petri dish (as described in [59]). The length of the axillary buds was monitored over a 10 day period.rps10b-1caused a slight delay in inflorescence outgrowth in bothMAX2andmax2-1backgrounds (Figure 3a, solid lines).
rps10b-1suppresses some auxin-related phenotypes ofmax2-1.(a) Growth and auxin sensitivity of cauline axillary buds in isolation from the plant. Single cauline nodes with a bud smaller than 2 mm were excised from sterile plants and inserted between two agar slabs in a Petri dish. Time course of bud elongation in the absence (solid lines, Control) and in the presence of synthetic auxin (1-naphthaleneacetic acid at 1 μM added to the apical agar slab, dashed lines, NAA). Average ± SEM, n = 12– 20. (b) Polar auxin transport along 1.5 cm stem segments from the base of the primary inflorescence of 6-week-old plants. 1 μM14C-IAA was in contact with the apical end of the segment without (black bars) or with the polar auxin transport inhibitor naphthylphtalamic acid (NPA, at 1 μM, white bars) for 6 hours. The mean radioactivity extracted from the basal 5 mm of the segments ± SEM is shown (n = 23–24 minus NPA, n = 9-10 plus NPA). (c-j) Activity of the auxin response reporter DR5::GUS in hypocotyls. Hypocotyls were stained for GUS activity (blue), fixed, embedded, sectioned at 10 μm, and counterstained with ruthenium red. (c-f) Hypocotyl sections from 2-week-old seedlings grown in continuous light. (g-j) Hypocotyl sections from 9-week-old plants grown in short photoperiods. Scale bar in (c) for (c-f) and in (g) for (g-j) 100 μm.
rps10b-1does not restore strigolactone responses tomax2-1, but confers auxin-related phenotypes antagonistic to those ofmax2-1
In addition to increased branching, themax2-1mutant has a range of phenotypes associated with its strigolactone insensitivity. These include an elongated hypocotyl and overexpression of the strigolactone biosynthetic genesCAROTENOID CLEAVAGE DIOXYGENASE7(CCD7) andCCD8, which are feedback-downregulated by strigolactone signalling [52,55,56].In a hypocotyl growth inhibition assay,rps10b-1did not suppress the strigolactone insensitivity ofmax2-1(Additional file2: Figure S1). Furthermore,rps10b-1did not affect levels ofCCD7orCCD8transcript characteristic of theMAX2- ormax2-1-backgrounds (Additional file3: Figure S2). Therefore, the suppression ofmax2-1byrps10b-1is specific to axillary shoot growth and does not involve a global restoration of strigolactone responsiveness.
Auxin has been implicated in both axillary meristem initiation and outgrowth. Furthermore,max2-1,in common with other strigolatone mutants, displays a number of auxin-related phenotypes, which led to the hypothesis that strigolactones act by restricting polar auxin transport. We therefore assessed the effect ofrps10b-1on these auxin-related phenotypes. The outgrowth of wild-type buds is strongly delayed by apical supply of the synthetic auxin naphthalene acetic acid (NAA), butmax2-1axillary buds are resistant to this auxin effect [55,59,60] (Figure 3a). In the wild-type background,rps10b-1delayed outgrowth only slightly, similar to its effect in the absence of auxin. However, in combination withmax2-1, rps10b-1substantially delayed bud outgrowth, such that the outgrowth of double mutant buds on auxin-treated explants was nearly identical to wild-type buds. Thus,rps10b-1suppressesmax2-1bud auxin resistance.
A second auxin-related phenotype ofmax2-1is an increase in basipetal transport of radiolabeled auxin through primary inflorescence stem segments [55,60].We found thatrps10b-1did not affect this phenotype (Figure 3b). Rather, the mutation slightly increased the amount of auxin transported in bothMAX2andmax2-1backgrounds.
Third, the auxin response reporter construct DR5::GUS [61] has increased activity in the main shoot axis ofmax2-1plants [60], associated with increased amounts of auxin moving in the PATS [44].We found that this increase in DR5::GUS expression was partially suppressed inrps10b-1 max2-1. This effect was observed in hypocotyls from 2-week old seedlings (Figure 3c–f) as well as hypocotyls from 9-week-old short-day grown plants, which had undergone secondary thickening (Figure 3g–j). In theMAX2background,rps10b-1had little effect, with xylem-associated DR5::GUS activity possibly slightly increased. These differences in reporter activity do not simply reflect differences in bud activity, because the 2-week-old seedlings had not yet initiated axillary buds. In summary,rps10b-1partially rescued some of the auxin-related phenotypes ofmax2-1, indicating thatRPS10Bmay act by modulating auxin responsiveness or homeostasis.
rps10b-1in high-branching mutant backgrounds
To learn more about the mode ofRPS10Baction we assessed its genetic interactions with other known shoot branching regulatory genes. First, the effect of therps10b-1mutation in high-branching mutant backgrounds other thanmax2-1was assessed (Figure 4a). As expected, the strigolactone biosynthetic mutantmax4-1(ccd8)[62], was partially suppressed.brc1-2andbrc2-1are loss of function alleles of bud-specific class IITCPtranscription factor genes [28].As withmax2, excessive branching ofbrc1-2is strigolactone-insensitive [53].brc1-2and thebrc1-2 brc2-1double mutant were also partially suppressed byrps10b-1. In all tested double and triple mutant combinations ofrps10b-1withmax4,brc1orbrc2, empty axils were present at apical nodes in the rosette at maturity. Thus, as withmax2, at least part of the suppression byrps10b-1in these backgrounds resulted from a defective or delayed axillary shoot formation.
Interaction ofrps10b-1with high-branching mutants.(a) Effect ofrps10b-1on the number of rosette branches at maturity inmax2-1, max4-1,brc1-2, brc1-2 brc2-1,amp1-1andaxr1-3mutant backgrounds. Branches ≥ 0.5 cm were counted, average ± SEM, n = 8-10. (b-g) Rosette centres of selected genotypes from (a), seen from above at early flowering stage. The primary inflorescences were between 3.0 and 3.4 cm long and were removed to reveal the rosette leaf axils. Scale bar in (b) for (b-g) 5 mm. (h) Therps10b-1bud initiation defect at apical rosette axils is rescued in therps10b-1 amp1-1double mutant. Quantitative analysis of rosette axillary shoot development at the reproductive stage was carried out as in Figure 2h, but only the topmost six rosette leaf axils of each plant were scored, n = 10-11.
Perception of auxin by the TIR1/AFB auxin receptors triggers the ubiquitin-mediated degradation of Aux/IAA proteins, which are repressors of the AUXIN RESPONSE FACTOR (ARF) transcriptional regulators [63].This degradation requires the AUXIN-RESISTANT1 (AXR1) protein [63,64].Mutation ofAXR1has little effect on bud initiation, but results in increased and auxin resistant bud outgrowth [65,66].In combination withrps10b-1, theaxr1-3mutant allele surprisingly enhanced the suppression of axillary bud development at both apical rosette (Figure 4b–e) and cauline nodes. In some experiments, as shown in Figure 4b–e, buds in these positions were considerably smaller than those of either single mutant. In other experiments, a large proportion of cauline and apical rosette axils appeared completely empty inrps10b-1 axr1-3plants. In addition, the primary inflorescence meristem ofrps10b-1 axr1-3plants frequently aborted. Between 20% and 50% of the double mutant individuals, but neither of the single mutants, had this phenotype. These observations suggest thatRPS10BandAXR1interact to promote shoot meristem development. However, this interaction appeared to be positionally restricted. At more basal rosette nodes of double mutant plants, bud behaviour resembled theaxr1single mutant; axillary buds initiated and formed inflorescence branches, such that rosette branch numbers ofaxr1-3andrps10b-1 axr1-3at maturity did not differ significantly (Figure 4a).
Mutation ofAMP1, which encodes a putative carboxypeptidase with unknown molecular function, causes a range of phenotypes related to shoot meristem function including constitutive axillary bud activation, increased shoot meristem size, increased rate of leaf initiation, and increased cytokinin content [67- - - - - -70].The defective axillary bud formation in apical rosette nodes typical ofrps10b-1was completely suppressed in anamp1-1background (Figure 4b, c, f–h), and at maturity, the average branch number ofrps10b-1 amp1-1plants did not differ significantly fromamp1-1plants. Genetic analysis by Vidaurre and coworkers [71] suggests a major function of ARF-mediated auxin signalling in embryogenic shoot meristem formation and vascularisation might be the downregulation of AMP1 activity. In the light of this finding, the genetic interaction withamp1-1further supports the idea that reduced ARF-mediated auxin signalling is involved in therps10b-1meristematic phenotypes.
rps10b-1in low-branching mutant backgrounds
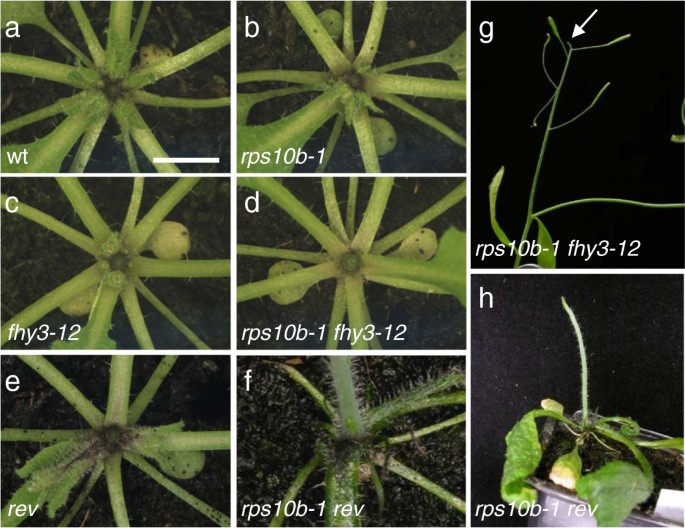
We also analysed the effect ofrps10b-1in genetic backgrounds characterised by reduced branching. First, we constructed a double mutant ofrps10b-1with another non-allelicmax2-1suppressor from our screen,far-red elongated hypocotyl3-12(fhy3-12). This is a loss-of function allele of the transcriptional activatorFHY3[72].This mutation suppressesmax2-1by reducing bud activation, with negligible effects on axillary shoot formation; and our data suggest that auxin might be central to its branching phenotype [73].rps10b-1 fhy3-12double mutant plants showed a near-complete loss of rosette axillary buds (Figure 5a–d). Furthermore, the primary inflorescence meristem of double mutant plants often aborted during the reproductive phase, a phenotype not observed with either single mutant (Figure 5g). The frequency of abortion ranged from 30% to 90% in different experiments.
Interaction ofrps10b-1with the low-branching mutantsfhy3-12andrev.(a-f) Rosette centres of wild-type, single and double mutant plants at early flowering stage. Except forrps10b-1 rev(SALK_102345), the primary inflorescences were removed to reveal the rosette leaf axils. Note the complete absence of rosette axillary buds inrps10b-1 fhy3-12andrps10b-1 rev, and the filament-like lateral organs at the top of therps10b-1 revrosette which are likely the youngest, radialised leaves. (g,h) Other shoot-meristem-related phenotypes of double mutant plants.(g)Abortion of the primary inflorescence meristem of anrps10b-1 fhy3-12plant in a short, pin-like structure (arrow). (h)rps10b-1 revprimary inflorescences were pin-like and lacked lateral organs.
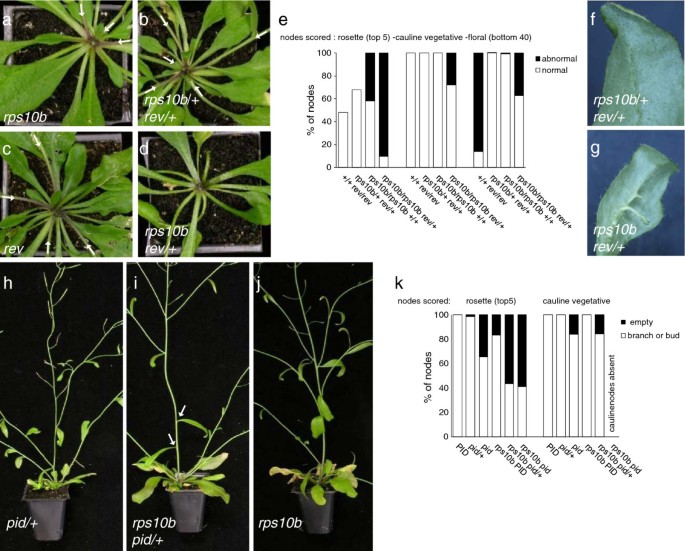
As described earlier, mutation of theHDZIPIIIgeneREVcauses partial defects in axillary meristem formation and floral meristem maintenance. In addition, theHDZIPIIIfamily members redundantly specify adaxial leaf identity, butrevloss-of-function mutant leaves appear normal [7,8].We generated double mutants betweenrps10b-1and arevT-DNA insertion allele, SALK_102345 (Figure 5f, h). These were highly abnormal. Successive leaves became increasingly needle-like, and axillary shoots were absent. The primary stem was short, pin-like and lacked flowers. Thus,rps10b-1strongly enhanced the loss ofREVfunction with respect to both leaf polarity and axillary shoot formation. The F2analysis also revealed that a single copy of therevmutant allele strongly enhanced the axillary shoot phenotypes in therps10b-1mutant background, whilerps10b-1/+ rev/+axillary shoot development was normal (Figure 6安妮). rps10b-1 rev/+plants had normal stature and slight defects in floral meristem maintenance. Their leaf polarity appeared largely normal, except that a few leaves had reduced lamina, from which the midvein separated as an abaxial outgrowth at the distal end of the leaf (Figure 6f, g). The strongest effect ofREVhaploinsufficiency concerned axillary shoot formation. Nearly all the rosette and a substantial proportion of cauline leaf axils were empty (Figure 6d, e). This demonstrates a dosage dependence ofREVin therps10b-1background, which is not seen in the wild-typeRPS10Bbackground, whererevappeared recessive.
Dosage effects ofREVandPIDin therps10b-1mutant background.(a-g) F2from a cross ofrps10b-1with therevmutant. (a-d) Rosettes of F2plants genotyped forRPS10BandREV. The primary inflorescences were removed. White arrows mark axillary shoots (branches or axillary buds). In the presence of functionalRPS10B(one copy inb, two copies inc), loss of one (b) or both (c) functionalREVcopies does not noticeably affect rosette branching. In therps10bmutant background (a,d) loss of one functionalREVcopy (d) completely abolishes axillary bud formation. (e) Quantification of axillary shoot development of genotyped F2隔离。玫瑰的比例,茎d floral nodes showing normal versus abnormal axillary shoot development are plotted. For vegetative nodes, development was classified as abnormal if the axil appeared empty. For floral nodes, development was classified abnormal when the node was occupied by a pedicel- or filament-like structure, and normal when it carried a flower or silique. For the rosette nodes ofrevandrps10b/+ rev/+plants only the number of branches was scored, thus the white bar represents a minimum estimate of the proportion of normal nodes and the proportion of abnormal nodes is not given. 7 to 21 individuals were scored per genotypic class. (f,g) Some leaves ofrps10b rev/+plants had outgrowths from the midvein at the abaxial side.rps10b/+ rev/+leaves appeared normal. (h-k) F3from a cross ofrps10b-1with thepid-14 T-DNA insertion allele (SALK_049736). Arps10b pid-14/+segregant with two basal nodes lacking axillary shoots (i, white arrows), occurrence of this phenotype inpid-14/+controls (h) was negligible and inrps10bcontrols (j) less frequent (seek). (k) Quantification of cauline and rosette axillary shoot development of genotyped F3隔离。Analysis was done as in (e). 7 to 16 individuals were scored per genotypic class.
ThePIDprotein kinase is required for dynamic changes in plasma-membrane localisation of PIN auxin transporters and thereby auxin transport direction [32- - - - - -34,74].Plants homozygous for strongpidmutant alleles are defective in flower formation, and the few flowers produced are abnormal and sterile [75].We crossedrps10b-1with apid-14heterozygote (SALK_049736 [34,76]) and homozygous double mutants were identified in segregatingrps10b-1 pid-14/+F3families (Figure 6h–k). In addition to the defect in flower formation, which has been described, homozygouspid-14segregants fromRPS10B pid-14/+control F3families showed mild defects in cauline and axillary bud initiation similar to therps10b-1single mutant.pid-14heterozygotes from the control F3were indistinguishable fromPIDsegregants and wild-type controls. The double mutants segregating in the progeny ofrps10b-1 pid-14/+plants had a more severe phenotype thanpid-14alone, as neither cauline leaves nor branches, nor flowers were produced on the primary inflorescence, and the proportion of empty rosette axils was increased. Furthermorerps10b-1 pid-14/+F3individuals also showed slightly enhanced axillary shoot defects when compared withrps10b-1 PIDF3segregants orrps10b-1controls. The proportions of empty cauline and rosette axils were increased (Figure 6k). Although less striking than withREV, there is aPIDdosage effect in therps10b-1background, demonstrating that partial loss of this r-protein increases sensitivity to reduced function of both PID and REV.
RPS10BsupportsCUCgene function
As described above,rps10b-1caused failure of the primary shoot meristem or of floral meristems in some mutant backgrounds. This could point to a more general role ofRPS10Bin supporting shoot meristematic function, which is also indicated by other weakly penetrant traits observed with therps10b-1single mutant. Inrps10b-1flowers, the number, identity and separation of lateral organs were affected (Figure 7). Sepal, petal, stamen and carpel numbers were more variable than in the wild type (Table 2). A substantial proportion ofrps10b-1flowers lacked one stamen, while petal and carpel numbers were more often increased than decreased (Figure 7a–d). Fusion between organs in one whorl was sometimes detected, most frequently for the stamens (Figure 7e). Furthermore, some stamens were green and possibly carpelloid (Figure 7f) and/or were partly fused to the gynoeceum (Figure 7g–h).
rps10b-1floral organ phenotypes.(a-d) Increased variation in floral organ number. Wild-type flower with four (a),rps10b-1flower with five petals (b). Developing siliques of wild type (c) with two carpels,ofrps10b-1(d) with three carpels. (e,g,h) Defective organ separation. Fusion of two stamen filaments indicated by black arrow in (e). Fusion of stamen filaments to the gynoeceum marked by arrowheads in (g,h). (f,g,h) Mis-specification of organ identity. Stamens in (f, white arrow), (g) and (h) showing carpelloid features. Scale bar in (h) for (a-h): 2 mm.
Furthermore, patterning defects in addition to the lack of axillary shoots were observed at cauline nodes at low frequencies (Figure 8a–c, Table 1). The topmost cauline branches ofrps10b-1were occasionally not subtended by a cauline leaf (Figure 8c), and fusion of cauline leaf lamina to the inflorescence stem was sometimes detected (Figure 8).
Genetic interaction betweenRPS10BandCUC3in organ separation.(a-f) Cauline vegetative nodes.cuc3(GABI-KAT GK_302G09) nodes are phenotypically wild-type (compareaandd), with rare exceptions, as inewhere acuc3accessory axillary bud (arrow) appeared to be fused with an axillary branch.rps10b-1nodes occasionally show leaf-to-stem fusion (arrow inb) or the cauline leaf is missing (c), in addition to the lack of the axillary bud which was shown in Figure 2f,g. The upper of the tworps10b-1 cuc3double mutant nodes infshows leaf-to-stem fusion (arrow). The bottom node lacks the cauline leaf and the bottom of the axillary branch may be fused with the primary inflorescence.Scale bar in (f) for (a-f): 5 mm.
Such phenotypes suggest a role ofRPS10Bin lateral organ partitioning and separation. To test this hypothesis, we studied the genetic interaction betweenRPS10BwithCUC3, one of three NAC transcription factor family members with partially overlapping roles in organ boundary formation. Anrps10b-1 cuc3double mutant was constructed using a T-DNA knockout allele ofcuc3(GABI-KAT line GK_302G09 [77]). With respect to cauline node development (Table 3and Figure 8a, d, e),cuc3was nearly indistinguishable from wild type, consistent with previous reports, demonstrating redundancy in the CUC family for cauline node patterning [11,12].Very rarely, we observed that accessory axillary shoots, which are often formed at Arabidopsis cauline nodes between the axillary branch and its subtending leaf (Figure 2e), were fused with the stem of the axillary branch (Figure 8e), or that a branch was slightly fused with the base of its subtending cauline leaf. In contrast, in therps10b-1 cuc3double mutant, the frequency of obvious cauline node patterning defects was greatly enhanced. There was further loss of either the leaf or the axillary shoot, and increased fusion of organs, such that 76% of the double mutant nodes appeared abnormal (Table 3). The increase in the proportion of nodes showing abnormal leaf development (leaf absent or fused to the stem) in the double mutant, compared withrps10b-1alone, was highly significant (χ2 = 113.1, p < 0.0001). This was also the case when the proportions of nodes lacking an axillary shoot were compared (χ2 = 72.3, p < 0.0001).
Loss ofCUC3function has been reported mildly to affect embryonic shoot patterning, withcuc3seedlings falling into two major classes: phenotypically normal, or showing one-sided cotyledon fusion. Occurrence of the severe cup-shaped phenotype caused by two-sided cotyledon fusion is rare [10,11].This was also true for thecuc3allele we used (Table 4).rps10b-1single mutant seedlings did not show cotyledon fusion but rarely, an extra cotyledon was present. Combiningrps10b-1andcuc3doubled the proportion of seedlings showing cotyledon fusion (13.9%, compared to 7.7% forcuc3alone, χ2 = 6.77, p = 0.01). It also increased the proportion of seedlings showing severe, two-sided cotyledon fusion, but not significantly (Fisher’s exact test, p = 0.06). Thus, the patterning of cotyledonary nodes appeared less sensitive to combined loss ofRPS10BandCUC3function than the patterning of cauline nodes. Our observations suggest thatCUCgene-mediated patterning depends on fullRPS10Bfunction, but also that this dependence varies with the developmental context.
Functional redundancy of RPS10B and RPS10C in the control of development
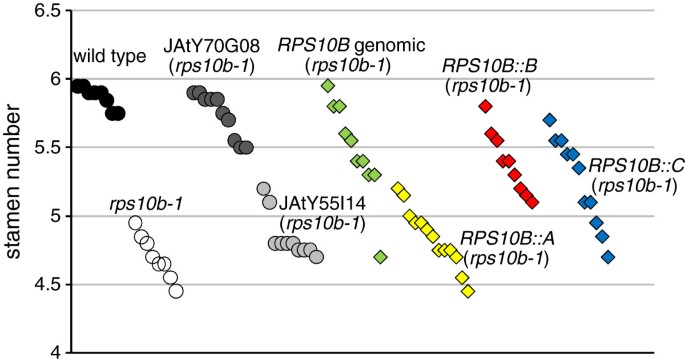
Arabidopsis r-proteins are encoded by small gene families [78].Two additionalRPS10family members,RPS10A(At4g25740) andRPS10C(At5g52650), show 78% and 74% amino acid identity withRPS10B. RT-PCR from cDNA produced from total RNA of different wild-type tissues showed that all three genes are transcribed and that their relative contributions to transcript level appear invariant for the tissues we analysed (Additional file4: Figure S3). The AtProteome database [79] points to RPS10B as the most abundant protein isoform. To test for redundancy of protein function, we amplified a cDNA corresponding to the longest annotated protein version for each member, and expressed it under the control of theRPS10Bpromoter inrps10b-1plants. As controls we used the wild type,rps10b-1, T1plants from transformation of the mutant with the genomicRPS10Bconstruct, and T2plants from transformation of the mutant with two JAtY TAC clones, only one of which contained theRPS10Bgenomic region. Complementation efficiency was scored by counting the stamens of 20 flowers from 8–13 individual plants per genotype or construct (Figure 9). The mean individual stamen numbers ranged between 5.7 and 6 for wild-type plants; but were below 5.4 for the mutant or transformants with the JAtY TAC clone that lackedRPS10B. For the T2transformed with the JAtY TAC containingRPS10B, and for 9 out of 10 T1转化的基因RPS10Bconstruct, stamen numbers ranged from 5.4 up to the maximum values obtained for wild-type plants. A mean stamen number lower than wild type but still above those of mutant plants may be explained by a lower dose of functionalRPS10Bin some transformants than in wild type, as the majority of the JAtY T2and most of the T1are expected to contain one transgene copy. Of the threeRPS10Bpromoter::cDNA fusions,RPS10B::Brescued most efficiently, however with a further reduction compared to the genomic construct, which could indicate a requirement to generate alternative transcripts, or for intronic or untranslated sequences for the proper control ofRPS10Bgene expression. TheRPS10B::C构建补充abo血型的雄蕊表型ut half of the T1; however, none of theRPS10B::AT1was rescued. While the reason for the non-complementation byRPS10Ais not clear, the rescue by theRPS10CcDNA argues against a specialised role of RPS10B within the S10e protein family.
Assessing functional redundancy of RPS10 proteins.RPS10Bpromoter::RPS10cDNA fusions were constructed for theRPS10family membersA,BandCand transformed intorps10b-1. The T1was scored for rescue of reduced stamen number, anrps10b-1developmental phenotype. Controls: wild type,rps10b-1, transgenic T2plants from transformation ofrps10b-1with JAtY TAC clone 70 G08 which spanned, and clone 55I14 which lacked theRPS10Bgenomic region; and T1plants from the transformation ofrps10b-1with a genomicRPS10Bclone. Each symbol represents the mean stamen number of 20 flowers from the primary inflorescence of one individual plant.
Discussion
TheRPS10gene family
RPS10Bbelongs to the three-member Arabidopsis gene family encoding the eukaryote-specific protein S10e of the small cytoplasmic ribosomal subunit [78,80].Like most of the r-proteins, S10e is essential for the biogenesis of its ribosomal subunit [81].It is positioned at the “beak” of the small subunit, a structure that is formed from protein and rRNA in eukaryotes, but exclusively from rRNA in bacteria [82].The role of S10e in translation is unknown. Crosslinking experiments indicate that S10e might participate in the interaction of the small subunit with eukaryotic initiation factor 3, which functions in translation initiation [83,84].
The Arabidopsisrps10b-1mutant allele is transcribed and can encode a truncated protein; its recessive inheritance is consistent with either reduced or abolished protein function. A knockout allele could not be obtained from T-DNA mutant collections. The fact that cDNAs ofRPS10BandRPS10C(driven by theRPS10Bpromoter) rescued anrps10b-1mutant phenotype to a similar extent, suggests that the RPS10B protein has not functionally diverged from other family members. We detected transcripts of allRPS10family members in all tissues tested, with highest transcript levels in young, growing tissues, including axillary buds (Additional file4: Figure S3).
The specificity of ribosomal protein mutant phenotypes
An increasing collection of ribosomal protein mutants have been recovered from screens for developmental phenotypes in Arabidopsis, with substantial overlap in the suite of phenotypes conferred by these mutations. The phenotypes include altered leaf shape (the first leaves are narrow and pointed) and the ability to enhance the phenotype of mutations that affect leaf adaxial identity, for exampleasymmetric leaves1(as1) oras2[85- - - - - -91].However, these r-protein mutations differ substantially in their effects on plant growth, which could reflect variation in the degree of genetic redundancy. Inrps10b-1, expression of the pointed first leaf phenotype was mild.Leaf polarity was affected in double mutant combination withrev(Figures 6,7) and we confirmed that this was also the case withas1(Additional file5: Figure S4). Although we observed weak effects on growth rate, for example in axillary buds on isolated nodal segments, the shoot or organ size of mature plants was not noticeably reduced, arguing against a general growth defect. The basis of the developmental defects of r-protein mutants is unclear. Two possibilities seem likely.
First, defective ribosomes may trigger specific developmental defects through their participation in surveillance mechanisms at cell cycle checkpoints. For example in humans, redundancy of r-proteins is less common, and haploinsufficiency of S10e and several other proteins of the large or small ribosomal subunit cause Diamond-Blackfan anemia, a syndrome of specific developmental defects including the failure of red blood cell progenitors [92,93].According to current understanding of the disease, these mutations perturb ribosome biogenesis via an imbalance in ribosome constituent stoichiometry. This is likely to increase the level of unincorporated r-proteins, several of which can bind and inactivate a ubiquitin ligase which targets the p53 tumor suppressor protein [94,95], and its resulting stabilization triggers cell cycle arrest in red blood cell progenitors. It may be that this surveillance mechanism operates in certain cell types only, for example cells that proliferate very rapidly [96,97], which could explain the developmental specificity of the phenotype. It is not known whether similar surveillance systems operate in plants.
Second, ribosome insufficiency, the production of disfunctional ribosomes, or the lack of ribosomes containing a specific r-protein variant could affect the production of specific proteins more than others. For example developmental patterning or cell cycle genes might crucially depend on particularly high translation rates or on a specialized ribosome variant. An interesting case here is the Aux/IAA transcriptional repressors, which are central to auxin-regulated gene expression. Some members of this protein family have extremely short half-lives, in the order of 5 minutes [98], and are maintained at steady state level in cells with a particular auxin concentration. Upon auxin addition, their half-lives are further reduced [64,99], resulting in their depletion and hence the up-regulation of transcription by a sub-family of ARFs. Because of the need for continuous replenishment of these proteins, it is possible that developmental events dependent on dynamic changes in auxin signaling are particularly sensitive to inefficient ribosomes. Alternatively, the consequences of reduced or altered ribosome function might be enhanced by specific features of the mRNA encoding a protein, for example by the presence of upstream ORFs, which require translation re-initiation. This is the case for the mRNAs of several ARF transcription factors, including ARF3 (ETTIN) and ARF5 (MONOPTEROS, MP), and was proposed to causearf-like developmental phenotypes of the r-protein mutantshort valve1(rpl24b) [100].Another ribosome-dependent process, which might potentially be affected is miRNA-directed translational regulation [101,102].Many of the genes involved in meristem patterning and adaxial identity are regulated by small RNAs [103,104].
The work presented here is suggestive of this second set of possibilities, because many of the effects we observe are indicative of a general lack of robustness of the adaxial patterning system, with therps10b-1mutation rendering the system sensitive to the dosage of other important regulatory components.
RPS10Band shoot meristem function
Despite the intuitive lack of specificity expected from a ribosomal protein mutation, it is clear that mutation ofRPS10Bcauses a syndrome of phenotypes that can be attributed to patterning events at the shoot apical meristem, and particularly to the establishment of boundaries between the meristem and the leaf, and to a lesser extent, within the leaf.
rps10b-1suppresses excessive shoot branching in themax2-1mutant background. A reduced ability to initiate or maintain axillary shoot meristems is a major cause of this suppression. The axillary shoot defects ofrps10b-1were enhanced in double mutant combination withaxr1,fhy3,rev, andpid, and were sensitive to reductions in the dose ofREVandPID. Moreover, maintenance of the primary shoot apical meristem was partially affected in combination withaxr1andfhy3; a phenotype not observed in the single mutants. Finally,rps10b-1enhanced the floral meristem defects ofrevandpid. This indicates a general role ofRPS10Bin shoot meristem function. In addition, therps10b-1 revdouble mutant phenotype revealed thatRPS10Bis involved in leaf polarity, like many other r-protein genes.
While axillary meristem defects have not yet been reported for r-protein gene single mutants (perhaps because they are relatively weak), introgression ofpiggy1(rpl10ab) into arevmutant,stv1(rpl24b) into anarf3mutant andrpl4dinto anas1mutant background resulted in striking axillary and/or floral meristem defects [88,100,105].Formation of the embryonic shoot meristem; and shoot meristem, vascular and leaf patterning crucially depend on an interaction betweenHDZIPIIIandKANgenes [2,19,106].Furthermore, axillary and embryonic shoot meristem formation are similar in many respects and likely to share theHDZIPIII /KANpatterning mechanism. With respect to leaf patterning, r-protein genes were found to promote genetically the adaxialising role of theHDZIPIIIgenes, and to antagonise the action of the abaxialisingKANgenes [105,107].RPS10Bgenetically promoted the action ofREVboth in shoot meristem function and leaf polarity. Interestingly, axillary meristem formation appeared more sensitive to halving theREVdose in therps10b-1background, than did leaf polarity. This supports the notion thatRPS10Bacts at least partly via meristem establishment itself, and not only via the specification of leaf adaxial fate, which is a prerequisite for axillary meristem initiation in Arabidopsis [5,7,8,16].Despite the strong genetic interactions between r-protein genes and theHDZIPIII/KANpathway, further analysis did not implicate any of the ad- or abaxial polarity genes examined as direct targets of ribosomal regulation [88,91,105,107].
Therps10b-1突变体显示其他拍摄meristem-related苯酚的otypes that were not enhanced in combination withrev. Cauline nodes lacked a leaf, or the leaf was rudimentary. Sometimes, the cauline leaf margin was fused to the stem. Floral organ numbers were more variable than in the wild type and, organ fusion within and between whorls occurred. Such phenotypes indicate misregulated organ separation. Somerps10b-1phenotypes resemble loss of function, while others resemble gain of function phenotypes described for the three partially redundantCUCgenes [9- - - - - -12,108- - - - - -110].Furthermore, combiningrps10b-1 andcuc3enhanced organ separation defects which were rare in the single mutants, most dramatically at cauline nodes. In the r-protein mutantrpl27ac-1d,CUC2was mislocalised during embryonic shoot meristem formation [91].Interestingly, the leaf polarity regulatorsAS1andAS2have been implicated inCUCgene regulation and organ boundary formation [111- - - - - -113].Conversely, mutant phenotypes suggest a role forCUCgenes in leaf polarity [11].This suggests that the well-known ribosomal regulation of leaf polarity, and the organ boundary role we describe here forRPS10Bcould have a shared molecular basis.
RPS10Band auxin
A common feature of many of the developmental events described above is their dependence on, or interaction with auxin and its directed transport. The formation of both the leaf-meristem boundary and the leaf abaxial-adaxial boundary involve the specific and dynamic reorientation of auxin transport paths and hence auxin distribution patterns [14].Consistent with the importance of auxin in these events, the general reduction in the robustness of the patterning of these boundaries in therps10b-1mutant is associated with a range of auxin-related phenotypes.
First, mutations affecting auxin signalling or transport enhanced some of these defects. The auxin signalling mutationaxr1-3, which does not affect axillary shoot formation in a wild-type background [66], enhanced axillary bud loss in combination withrps10b-1. In addition, a novel phenotype of primary inflorescence meristem arrest was displayed by someaxr1-3 rps10b-1double mutant plants. The effects of reduced or abolished function of the auxin transport regulatorPIDon lateral organ formation were enhanced in therps10b-1mutant background. The effect of both these auxin-related mutations may be to interfere withARF- - - - - -regulated developmental programmes either globally (axr1) or through altered auxin distribution (pid). Mutation of the transcriptional activatorFHY3, anothermax2branching suppressor from our screen, very strongly enhanced axillary meristem failure when combined withrps10b-1, and also caused inflorescence meristem arrest. We hypothesise thatFHY3also regulates branching via auxin signalling or homeostasis [73].
Second, theamp1-1mutation suppressed the axillary meristem failure ofrps10b-1in the double mutant. Although the molecular function of AMP1 is not known, loss-of-function mutant phenotypes suggest that it restricts shoot meristematic growth [70,114].Increased levels of cytokinins have been detected inamp1plants [67,68], which might cause their increased meristematic stem cell activity [115].Interestingly, a link betweenAMP1andARF-mediated auxin signaling has recently been proposed.amp1suppresses the effect of loss ofARF5(MP) in embryonic shoot meristem development and vascularisation, indicating that one important activity ofMPmight be to antagoniseAMP1[71].In this way, auxin signalling in the shoot meristem could sustain the stem cell pool required for future lateral organ formation. The genetic interactions ofrps10bwithaxr1,pid, andamp1are consistent withRPS10Bsupporting stem cell production indirectly by maintainingARF-mediated auxin signalling.
RPS10B and axillary bud outgrowth
In addition to axillary meristem specification defects, which likely underlie the poor axillary shoot formation phenotype of therps10b-1mutant, we also detected defects in axillary meristem activity, which may contribute to the suppression of shoot branching inmax2. Because of the effects on axillary bud formation, it was difficult to ascertain the effect ofrps10bon bud outgrowth in intact plants. Therefore, we used excised cauline nodes, which were selected for approximately equal bud size at the start of the experiment. Except for one specific situation, which is discussed below, the effect ofrps10bon bud outgrowth rate was surprisingly small, given the transcriptional evidence for high r-protein synthesis in active buds [25,26].This could indicate that loss ofRPS10Bwas compensated by functional family members. Mechanisms that ensure that ribosomal components are produced in stochiometric amounts are better studied in other organisms, but they are likely to operate in plants as well [116,117].We detected at most a slight upregulation ofRPS10AorCtranscripts inrps10bby semi-quantitative RT-PCR (Figure 1f). However, the example of the Arabidopsisrpl4aandrpl4dmutants shows that compensation at the protein level can occur in the absence of detectable compensation at the transcript level [118].
The F-box protein MAX2 is required for normal strigolactone responsiveness, and is thought to act in an E3 ubiquitin ligase, selecting unknown protein targets for degradation [119,120].Strigolactones are negative regulators of PIN protein levels, and of polar auxin transport in the vasculature [55].Recent studies with excised axillary buds, to which a synthetic strigolactone was applied via the basal internode yielded two interesting observations. First, the ability of strigolactone to inhibit single excised buds required apical auxin; second, if buds on two consecutive nodes were excised, basal strigolactone enhanced the growth differential or competition between them, rather than inhibiting both [55,121].This fits with a model of bud regulation via auxin transport canalisation, where bud activation requires the export of auxin via a shared auxin transport route in the stem, and strigolactones inhibit this process by restricting PIN protein accumulation [45].Consistent with this, axillary buds of strigolactone mutants, includingmax2, are moderately resistant to apically applied auxin [55,60].Interestingly, we found that the growth-inhibiting effect ofrps10bon auxin-treatedmax2buds was much stronger than for other genotypes and treatment combinations, such that bud outgrowth kinetics of the auxin-treated double mutant were restored to wild-type. This could indicate thatrps10bspecifically suppresses a downstream effect of themax2mutation in bud outgrowth. This effect might be auxin-related, asrps10bspecifically suppressed auxin responsive gene expression, as reported by DR5::GUS activity, in the shoot axis ofrps10b max2, while it did not have this effect in theMAX2background. A mode of action different from strigolactone / MAX2 is suggested by the fact thatrps10bdid not antagonise the effect ofmax2on stem polar auxin transport; and the fact thatrps10bdid not restore the altered shoot vascular architecture ofmax2back to wild type (compare sections of older plants in Figure 3g-j). The vasculature ofmax2stems shows increased activity of the PIN1::PIN1-GFP reporter [55,60].In a recent evaluation of the vascular role of theHDZIPIIIandKANgenes, both contributed to focused and canalised auxin movement during vascular differentiation; it was proposed thatKANgenes act by downregulating PIN activity, and thatHDZIPIIIs promote the differentiation of xylem tissues, including the auxin-conducting xylem parenchyma [42].A relatively subtle change in the HDZIPIII / KAN activity balance characteristic for the r-protein mutants, with lowered HD/ZIPIII or increased KAN activity, might not be critical for bud auxin export and activation in wild type, but might prevent buds ofmax2from activating when there is a higher auxin load in the main stem.
Conclusions
Our analysis of RPS10B function suggests a role in patterning and in boundary establishment at the shoot apex, processes that are intimately connected with dynamic regulation of auxin flows. Furthermore, RPS10B is required to sustain the outgrowth ofmax2腋窝味蕾的生长素,而我s largely dispensable for bud outgrowth otherwise. Regulation of development is not likely to be a specialised role of RPS10B within the S10e protein family. Howeverrps10b-1and other r-protein mutants highlight the importance of ribosomal function for normal development. Combined with advances in the study of ribosomal activities [122], they might in the future help us to understand how plant ribosomal biogenesis and translation are controlled and integrated with development and growth.
Methods
Plants and growth conditions
Ecotype Col-0 was used as the wild-type control, and unless stated otherwise mutant lines were in this genetic background. The following lines were described previously:amp1-1[67,69],axr1-3[65,123],brc1-2andbrc2-1(SALK_091920 and SALK_023116 [28]),fhy3-12[73],max2-1[54],max4-1[62] andpid-14[34,76].Two lines obtained from T-DNA mutant collections were characterised by sequencing from both T-DNA borders: SALK_102345, an insertion in the last exon ofREV(At5g60690)上游的终止密码子,GABI-KAT line GK_302G09, an insertion affecting the second exon ofCUC3(At1g76420). Multiple mutants which we constructed were confirmed by genotyping, using wild-type and T-DNA allele-specific PCR for insertional alleles, and CAPS [124] or dCAPS [125] markers for point mutation alleles; except formax4-1,where homozygosity was confirmed by testing progeny for uniform BASTA-resistance. AsREVandRPS10Bare linked, a reduced frequency of double mutant individuals was expected in the F2of therps10bxrevcross. Therefore, 36rps10b-1homozygous F2were selected based on their seedling leaf phenotype, genotyped forRPS10BandREV,他们的叶子和横向发展observed. For the crossrps10bxpid-14, genotyping was used in the F2to identifyrps10b-1 pid-14/+individuals expected to segregate the double mutant in the F3, withRPS10B pid-14/+individuals used as controls. About 40 F3progeny each were then genotyped and phenotyped.
Arabidopsis seeds were sown onto Levington F2 compost pretreated with systemic insecticide (Intercept 70WG, Everris Limited, Ipswich, UK). Trays were chilled at 4°C for 3 days and then incubated in a greenhouse with 16-h supplemental lighting. These conditions were used for all soil-grown plants except for the hypocotyls examined by histology (Figure 3). These were from 14-day-old plants grown in continuous low light (40 μmoles m-2 sec-1from white fluorescent tubes, 21°C) and from 60-day-old plants grown in short (8-h) photoperiods (160 μmoles m-2 sec-1from fluorescent white tubes, 21°C day / 17°C night temperature). Except for the mutant screen described below, individual plants were grown at a density of 1 per 16 cm2in trays consisting of 40 x 16 cm2compartments.
Identification of RPS10B as amax2-1suppressor
max2-1seeds were mutagenised with 0.3% ethyl methanesulfonate. 18 000 seeds from the resulting M2generation were sown at densities of one plant per 3 or 5 cm2and screened for reduced rosette branching at maturity. One of the suppressor mutations isolated,6-7, was recessive and segregated independently frommax2-1after backcrossing to Columbia wild-type. The suppressor locus was mapped to a 126-kbp interval on Chromosome 5 using about 1600 mutant individuals from the F2of a cross between Lerplants and the67mutant in theMAX2background. End-sequenced TAC clones from the Arabidopsis wild-type Columbia genomic JAtY library in pYLTAC17 [57] with inserts spanning the mapping interval were obtained from the John Innes Genome Laboratory, and transformed into Agrobacterium strain GV3101 for floral dipping of6-7 MAX2. This was done according to Clough and Bent [126], except that the infiltration medium contained glucose instead of sucrose. T1selected for BASTA-resistance under sterile conditions were further cultivated on soil. Their phenotypic rescue was scored; and they were genotyped to confirm the presence of the left and right vector – genomic insert borders specific to the TAC clone.
RNA isolation, RT-PCR, cloning
Total RNA was extracted using the RNeasy plant miniprep kit with on-column DNaseI digestion (Qiagen, Hilden, Germany) from about 100 mg tissue powder, obtained from 10 pooled 1-cm primary inflorescence stem base segments per genotype, from bolting plants of about 25 cm height. cDNA synthesis was performed from 1 μg total RNA in a total volume of 10 μl with SuperscriptII (Invitrogen, Life Technologies, Carlsbad, CA) and oligo-dT primer. After diluting each sample by adding 70 μl of water, 2 μl were used in 50 μl semi-quantitative PCR reactions with 26 cycles, unless stated otherwise. Gene-specificRPS10A-,RPS10B-andRPS10Cprimer pairs were used. RT-PCR forACTIN2(At3g18780) was used as RNA input control. Primer sequences are listed in Table 5.
A genomicRPS10Bconstruct was produced by amplifying a 3.5 kb fragment spanning theRPS10Bgenomic region from Columbia wild-type genomic DNA with primers RPS10BgenomicF and RPS10BgenomicR (Table 5). This was digested with SpeI and HindIII and cloned into binary vector pCAMBIA2300 (http://www.cambia.org) opened with XbaI and HindIII for plant transformation.
To expressRPS10A, RPS10B, RPS10CcDNAs under theRPS10Bpromoter,RPS10Bpromoter region was amplified from Columbia wild-type genomic DNA, and theRPS10A (At4g25740.1), RPS10B(At5g41520.1) andRPS10C(At5g52650.1) coding regions were amplified from Columbia wild-type cDNA using the primers specified in Table 5. The three forward primers for theRPS10coding regions introduced an overlap with theRPS10B上游启动子扩增子,然后融合of each cDNA by overlap extension in a second round of polymerase chain reaction. Furthermore, the primers introduced a BamHI site followed by a NotI site just upstream of the promoter and an XbaI site just downstream of the termination codon. The products were digested with BamHI and XbaI and ligated into the cloning vector pART7 [127] opened with the same enzymes. Inserts were confirmed by sequencing. From these plasmids, NotI releases a fragment consisting of theRPS10Bpromoter, theRPS10A,BorCcoding region and the plasmid-encoded octopine synthase gene terminator, which was transferred into a NotI-digested derivative of the plant transformation vector pART27 [127] which confers BASTA-resistance in plants. Confirmed constructs were shuttled into Agrobacterium strain GV3101 and used for plant transformation [126].
生长素的生理机能和运输、组织学hypocotyl sections stained for DR5::GUS activity
Axillary bud outgrowth assays were performed with cauline nodes excised from the primary inflorescence of plants grown in sterile conditions, as described [59].2-14C-indoleacetic acid transport assays were conducted with 1.5-cm stem segments from the basal internode of the primary inflorescence of 6-week old soil-grown plants [55,60].
2 mm of apical hypocotyl tissue and the cotyledonary node of 14 day-old seedlings germinated under continuous illumination and 2-mm segments from the thickened hypocotyls of 60-day-old short-day-grown plants were stained for β-glucuronidase (GUS) activity at 37°C overnight, fixed for 5 h, and embedded in Technovit (Heraeus Kulzer, Hanau, Germany); 10 μm transverse sections were prepared, mounted to slides, counterstained with ruthenium red, and permanently mounted as described [119].
References
- 1.
Long J, Barton MK: Initiation of axillary and floral meristems inArabidopsis. Dev Biol. 2000, 218: 341-353. 10.1006/dbio.1999.9572.
- 2.
Barton MK: Twenty years on: the inner workings of the shoot apical meristem, a developmental dynamo. Dev Biol. 2010, 341: 95-113. 10.1016/j.ydbio.2009.11.029.
- 3.
Keller T, Abbott J, Moritz T, Doerner P:Arabidopsis REGULATOR OF AXILLARY MERISTEMS1controls a leaf axil stem cell niche and modulates vegetative development. Plant Cell. 2006, 18: 598-611. 10.1105/tpc.105.038588.
- 4.
Müller D, Schmitz G, Theres K:BlindhomologousR2R3 Mybgenes control the pattern of lateral meristem initiation inArabidopsis. Plant Cell. 2006, 18: 586-597. 10.1105/tpc.105.038745.
- 5.
Talbert PB, Adler HT, Parks DW, Comai L: TheREVOLUTAgene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems ofArabidopsis thaliana. Development. 1995, 121: 2723-2735.
- 6.
Otsuga D, DeGuzman B, Prigge MJ, Drews GN, Clark SE:REVOLUTAregulates meristem initiation at lateral positions. Plant J. 2001, 25: 223-236. 10.1046/j.1365-313x.2001.00959.x.
- 7.
Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL: Radial patterning ofArabidopsisshoots by class III HD-ZIP and KANADI genes. Curr Biol. 2003, 13: 1768-1774. 10.1016/j.cub.2003.09.035.
- 8.
Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE: Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell. 2005, 17: 61-76. 10.1105/tpc.104.026161.
- 9.
Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M: Genes involved in organ separation inArabidopsis: an analysis of thecup-shaped cotyledonmutant. Plant Cell. 1997, 9: 841-857. 10.1105/tpc.9.6.841.
- 10.
Vroemen CW, Mordhorst AP, Albrecht C, Kwaaitaal MACJ, de Vries SC: TheCUP-SHAPED COTYLEDON3gene is required for boundary and shoot meristem formation in Arabidopsis. Plant Cell. 2003, 15: 1563-1577. 10.1105/tpc.012203.
- 11.
Hibara K, Karim MR, Takada S, Taoka K, Furutani M, Aida M, Tasaka M:Arabidopsis CUP-SHAPED COTYLEDON3regulates postembryonic shoot meristem and organ boundary formation. Plant Cell. 2006, 18: 2946-2957. 10.1105/tpc.106.045716.
- 12.
Raman S, Greb T, Peaucelle A, Blein T, Laufs P, Theres K: Interplay of miR164,CUP-SHAPED COTYLEDONgenes andLATERAL SUPPRESSORcontrols axillary meristem formation inArabidopsis thaliana. Plant J. 2008, 55: 65-76. 10.1111/j.1365-313X.2008.03483.x.
- 13.
Takada S, Hibara K, Ishida T, Tasaka M: TheCUP-SHAPED COTYLEDON1gene ofArabidopsisregulates shoot apical meristem formation. Development. 2001, 128: 1127-1135.
- 14.
Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM: Patterns of auxin transport and gene expression during primordium development revealed by live imaging of theArabidopsisinflorescence meristem. Curr Biol. 2005, 15: 1899-1911. 10.1016/j.cub.2005.09.052.
- 15.
McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK: Role ofPHABULOSAandPHAVOLUTAin determining radial patterning in shoots. Nature. 2001, 411: 709-713. 10.1038/35079635.
- 16.
Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS:KANADIregulates organ polarity inArabidopsis. Nature. 2001, 411: 706-709. 10.1038/35079629.
- 17.
Eshed Y, Baum SF, Perea JV, Bowman JL: Establishment of polarity in lateral organs of plants. Curr Biol. 2001, 11: 1251-1260. 10.1016/S0960-9822(01)00392-X.
- 18.
Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL: Asymmetric leaf development and blade expansion inArabidopsisare mediated by KANADI and YABBY activities. Development. 2004, 131: 2997-3006. 10.1242/dev.01186.
- 19.
Izhaki,鲍曼杰:KANADI和III类HD-Zip gene families regulate embryo patterning and modulate auxin flow during embryogenesis inArabidopsis. Plant Cell. 2007, 19: 495-508. 10.1105/tpc.106.047472.
- 20.
Green KA, Prigge MJ, Katzman RB, Clark SE:CORONA, a member of the class III homeodomain leucine zipper gene family in Arabidopsis, regulates stem cell specification and organogenesis. Plant Cell. 2005, 17: 691-704. 10.1105/tpc.104.026179.
- 21.
Kim J, Jung JH, Reyes JL, Kim YS, Kim SY, Chung KS, Kim JA, Lee M, Lee Y, Narry KV, Chua NH, Park CM: microRNA-directed cleavage ofATHB15mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J. 2005, 42: 84-94. 10.1111/j.1365-313X.2005.02354.x.
- 22.
Williams L, Grigg SP, Xie M, Christensen S, Fletcher JC: Regulation ofArabidopsisshoot apical meristem and lateral organ formation by microRNAmiR166gand itsAtHD-ZIPtarget genes. Development. 2005, 132: 3657-3668. 10.1242/dev.01942.
- 23.
Aida M, Tasaka M: Genetic control of shoot organ boundaries. Curr Opin Plant Biol. 2006, 9: 72-77. 10.1016/j.pbi.2005.11.011.
- 24.
Bilsborough GD, Runions A, Barkoulas M, Jenkins HW, Hasson A, Galinha C, Laufs P, Hay A, Prusinkiewicz P, Tsiantis M: Model for the regulation ofArabidopsis thalianaleaf margin development. Proc Natl Acad Sci U S A. 2011, 108: 3424-3429. 10.1073/pnas.1015162108.
- 25.
Devitt ML, Stafstrom JP: Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol Biol. 1995, 29: 255-265. 10.1007/BF00043650.
- 26.
Tatematsu K, Ward S, Leyser O, Kamiya Y, Nambara E: Identification of cis-elements that regulate gene expression during initiation of axillary bud outgrowth in Arabidopsis. Plant Physiol. 2005, 138: 757-766. 10.1104/pp.104.057984.
- 27.
Martín-Trillo M, Cubas P: TCP genes: a family snapshot ten years later. Trends Plant Sci. 2010, 15: 31-39. 10.1016/j.tplants.2009.11.003.
- 28.
Aguilar-Martínez JA, Poza-Carrión C, Cubas P:Arabidopsis BRANCHED1acts as an integrator of branching signals within axillary buds. Plant Cell. 2007, 19: 458-472. 10.1105/tpc.106.048934.
- 29.
Finlayson SA: Arabidopsis TEOSINTE BRANCHED-LIKE 1 regulates axillary bud outgrowth and is homologous to monocot TEOSINTE BRANCHED1. Plant Cell Physiol. 2007, 48: 667-677. 10.1093/pcp/pcm044.
- 30.
Müller D, Leyser O: Auxin, cytokinin and the control of shoot branching. Ann Bot. 2011, 107: 1203-1212. 10.1093/aob/mcr069.
- 31.
Vernoux T, Besnard F, Traas J: Auxin at the shoot apical meristem. Cold Spring Harb Perspect Biol. 2010, 2: a001487-10.1101/cshperspect.a001487.
- 32.
Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R: The PINOID protein kinase regulates organ development inArabidopsisby enhancing polar auxin transport. Development. 2001, 128: 4057-4067.
- 33.
Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, Hooykaas PJJ, Palme K, Offringa R: A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004, 306: 862-865. 10.1126/science.1100618.
- 34.
Huang F, Zago MK, Abas L, van Marion A, Galván-Ampudia CS, Offringa R: Phosphorylation of conserved PIN motifs directsArabidopsisPIN1 polarity and auxin transport. Plant Cell. 2010, 22: 1129-1142. 10.1105/tpc.109.072678.
- 35.
Bowman JL, Floyd SK: Patterning and polarity in seed plant shoots. Annu Rev Plant Biol. 2008, 59: 67-88. 10.1146/annurev.arplant.57.032905.105356.
- 36.
Vernoux T, Brunoud G, Farcot E, Morin V, Van den Daele H, Legrand J, Oliva M, Das P, Larrieu A, Wells D, Guédon Y, Armitage L, Picard F, Guyomarch S, Cellier C, Parry G, Koumproglou R, Doonan JH, Estelle M, Godin C, Kepinski S, Bennett M, De Veylder L, Traas J: The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol. 2011, 7: 508-
- 37.
Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida M:PIN-FORMED1andPINOIDregulate boundary formation and cotyledon development inArabidopsisembryogenesis. Development. 2004, 131: 5021-5030. 10.1242/dev.01388.
- 38.
Zhou GK, Kubo M, Zhong R, Demura T, Ye ZH: Overexpression of miR165 affects apical meristem formation, organ polarity establishment and vascular development inArabidopsis. Plant Cell Physiol. 2007, 48: 391-404. 10.1093/pcp/pcm008.
- 39.
Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J: Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003, 115: 591-602. 10.1016/S0092-8674(03)00924-3.
- 40.
Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C: Regulation of phyllotaxis by polar auxin transport. Nature. 2003, 426: 255-260. 10.1038/nature02081.
- 41.
Wenzel CL, Schuetz M, Yu Q, Mattsson J: Dynamics ofMONOPTEROSandPIN-FORMED1expression during leaf vein pattern formation inArabidopsis thaliana. Plant J. 2007, 49: 387-398. 10.1111/j.1365-313X.2006.02977.x.
- 42.
Ilegems M, Douet V, Meylan-Bettex M, Uyttewaal M, Brand L, Bowman JL, Stieger PA: Interplay of auxin, KANADI and Class III HD-ZIP transcription factors in vascular tissue formation. Development. 2010, 137: 975-984. 10.1242/dev.047662.
- 43.
Li CJ, Bangerth F: Autoinhibition of indoleaceticacid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol Plant. 1999, 106: 415-420. 10.1034/j.1399-3054.1999.106409.x.
- 44.
Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O: Control of bud activation by an auxin transport switch. Proc Natl Acad Sci U S A. 2009, 106: 17431-17436. 10.1073/pnas.0906696106.
- 45.
Leyser O: Auxin, self-organisation, and the colonial nature of plants. Curr Biol. 2011, 21: R331-R337. 10.1016/j.cub.2011.02.031.
- 46.
李CJ,格瓦拉E, Herrera J, Bangerth F:效果apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol Plant. 1995, 94: 465-469. 10.1111/j.1399-3054.1995.tb00955.x.
- 47.
田中M,武井K,小岛,神H, Mori H:Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 2006, 45: 1028-1036. 10.1111/j.1365-313X.2006.02656.x.
- 48.
Sachs T, Thimann KV: The role of auxins and cytokinins in the release of buds from dominance. Amer J Bot. 1967, 54: 136-144. 10.2307/2440896.
- 49.
Foo E, Bullier E, Goussot M, Foucher F, Rameau C, Beveridge CA: The branching geneRAMOSUS1mediates interactions among two novel signals and auxin in pea. Plant Cell. 2005, 17: 464-474. 10.1105/tpc.104.026716.
- 50.
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, Rochange SF: Strigolactone inhibition of shoot branching. Nature. 2008, 455: 189-194. 10.1038/nature07271.
- 51.
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S: Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008, 455: 195-200. 10.1038/nature07272.
- 52.
Hayward A, Stirnberg P, Beveridge C, Leyser O: Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009, 151: 400-412. 10.1104/pp.109.137646.
- 53.
Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA: Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009, 150: 482-493. 10.1104/pp.108.134783.
- 54.
Stirnberg P, Van de Sande K, Leyser HMO:MAX1andMAX2control shoot lateral branching inArabidopsis. Development. 2002, 129: 1131-1141.
- 55.
Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O: Strigolactones enhance competition between shoot branches by dampening auxin transport. Development. 2010, 137: 2905-2913. 10.1242/dev.051987.
- 56.
Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y, Yamaguchi S, McCourt P: A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat Chem Biol. 2010, 6: 741-749. 10.1038/nchembio.435.
- 57.
刘YG, Nagaki K, Fujita M, Kawaura K, Uozumi M, Ogihara Y: Development of an efficient maintenance and screening system for large-insert genomic DNA libraries of hexaploid wheat in a transformation-competent artificial chromosome (TAC) vector. Plant J. 2000, 23: 687-695. 10.1046/j.1365-313x.2000.00827.x.
- 58.
Hempel FD, Feldman LJ: Bi-directional inflorescence development inArabidopsis thaliana: Acropetal initiation of flowers and basipetal initiation of paraclades. Planta. 1994, 192: 276-286. 10.1007/BF01089045.
- 59.
Chatfield SP, Stirnberg P, Forde BG, Leyser O: The hormonal regulation of axillary bud growth inArabidopsis. Plant J. 2000, 24: 159-169. 10.1046/j.1365-313x.2000.00862.x.
- 60.
Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O: The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol. 2006, 16: 553-563. 10.1016/j.cub.2006.01.058.
- 61.
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ: Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997, 9: 1963-1971.
- 62.
Sorefan K, Booker J, Haurogne K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, Leyser O:MAX4andRMS1are orthologous dioxygenase-like genes that regulate shoot branching inArabidopsisand pea. Genes Dev. 2003, 17: 1469-1474. 10.1101/gad.256603.
- 63.
Chapman EJ, Estelle M: Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet. 2009, 43: 265-285. 10.1146/annurev-genet-102108-134148.
- 64.
Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M: Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature. 2001, 414: 271-276. 10.1038/35104500.
- 65.
Lincoln C, Britton JH, Estelle M: Growth and development of theaxr1mutants ofArabidopsis. Plant Cell. 1990, 2: 1071-1080.
- 66.
Stirnberg P, Chatfield SP, Leyser HMO:AXR1acts after lateral bud formation to inhibit lateral bud growth in Arabidopsis. Plant Physiol. 1999, 121: 839-847. 10.1104/pp.121.3.839.
- 67.
Chaudhury AM, Letham S, Craig S, Dennis ES:amp1- a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant Journal. 1993, 4: 907-916. 10.1046/j.1365-313X.1993.04060907.x.
- 68.
Chin-Atkins AN, Craig S, Hocart CH, Dennis ES, Chaudhury AM: Increased endogenous cytokinin in theArabidopsis amp1mutant corresponds with de-etiolation responses. Planta. 1996, 198: 549-556.
- 69.
Helliwell CA, Chin-Atkins AN, Wilson IW, Chapple R, Dennis ES, Chaudhury A: The ArabidopsisAMP1gene encodes a putative glutamate carboxypeptidase. Plant Cell. 2001, 13: 2115-2125.
- 70.
Saibo NJM, Vriezen WH, De Grauwe L, Azmi A, Prinsen E, Van der Straeten D: A comparative analysis of theArabidopsismutantamp1-1and a novel weakamp1allele reveals new functions of the AMP1 protein. Planta. 2007, 225: 831-842. 10.1007/s00425-006-0395-9.
- 71.
Krogan NT Vidaurre DP, Ploense年代,Berleth老师:AMP1andMPantagonistically regulate embryo and meristem development inArabidopsis. Development. 2007, 134: 2561-2567. 10.1242/dev.006759.
- 72.
Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H: Transposase-derived transcription factors regulate light signaling inArabidopsis. Science. 2007, 318: 1302-1305. 10.1126/science.1146281.
- 73.
Stirnberg P, Zhao S, Williamson L, Ward S, Leyser O:FHY3promotes shoot branching and stress tolerance in Arabidopsis in anAXR1-dependent manner. Plant J. 2012, 10.1111/j.1365-313X.2012.05038.x. e-pub ahead of print
- 74.
Zhang J, Nodzyński T, Pĕnčík A, Rolčík J, Friml J: PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc Natl Acad Sci U S A. 2010, 107: 918-922. 10.1073/pnas.0909460107.
- 75.
Bennett SRM, Alvarez J, Bossinger G, Smyth DR: Morphogenesis inpinoidmutants ofArabidopsis thaliana. Plant J. 1995, 8: 505-520. 10.1046/j.1365-313X.1995.8040505.x.
- 76.
Cheng Y, Qin G, Dai X, Zhao Y: NPY1, a BTB-NPH3-like protein, plays a critical role in auxin-regulated organogenesis inArabidopsis. Proc Natl Acad Sci U S A. 2007, 104: 18825-18829. 10.1073/pnas.0708506104.
- 77.
Kleinboelting N, Huep G, Kloetgen A, Viehoever P, Weisshaar B: GABI-Kat SimpleSearch: new features of theArabidopsis thalianaT-DNA mutant database. Nucleic Acids Res. 2012, 40: D1211-D1215. 10.1093/nar/gkr1047.
- 78.
Barakat A, Szick-Miranda K, Chang IF, Guyot R, Blanc G, Cooke R, Delseny M, Bailey-Serres J: The organization of cytoplasmic ribosomal protein genes in the Arabidopsis genome. Plant Physiol. 2001, 127: 398-415. 10.1104/pp.010265.
- 79.
Baerenfaller K, Grossmann J, Grobei MA, Hull R, Hirsch-Hoffmann M, Yalovsky S, Zimmermann P, Grossniklaus U, Gruissem W, Baginsky S: Genome-scale proteomics revealsArabidopsis thalianagene models and proteome dynamics. Science. 2008, 320: 938-941. 10.1126/science.1157956.
- 80.
Lecompte O, Ripp R, Thierry JC, Moras D, Poch O: Comparative analysis of ribosomal proteins in complete genomes: an example of reductive evolution at the domain scale. Nucleic Acids Res. 2002, 30: 5382-5390. 10.1093/nar/gkf693.
- 81.
Ferreira-Cerca年代,调查G, Gleizes PE、Tschochner H, Milkereit P: Roles of eukaryotic ribosomal proteins in maturation and transport of pre-18S rRNA and ribosome function. Mol Cell. 2005, 20: 263-275. 10.1016/j.molcel.2005.09.005.
- 82.
Rabl J, Leibundgut M, Ataide SF, Haag A, Ban N: Crystal structure of the eukaryotic 40Sribosomal subunit in complex with initiation factor 1. Science. 2011, 331: 730-736. 10.1126/science.1198308.
- 83.
Westermann P, Nygård O: The spatial arrangement of the complex between eukaryotic initiation factor eIF-3 and 40S ribosomal subunit. Crosslinking between factor and ribosomal proteins. Biochim Biophys Acta. 1983, 741: 103-108. 10.1016/0167-4781(83)90015-5.
- 84.
Hinnebusch AG: eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem Sci. 2006, 31: 553-562. 10.1016/j.tibs.2006.08.005.
- 85.
Byrne ME: A role for the ribosome in development. Trends Plant Sci. 2009, 14: 512-519. 10.1016/j.tplants.2009.06.009.
- 86.
Creff A, Sormani R, Desnos T: The two ArabidopsisRPS6genes, encoding for cytoplasmic ribosomal proteins S6, are functionally equivalent. Plant Mol Biol. 2010, 73: 533-546. 10.1007/s11103-010-9639-y.
- 87.
Falcone Ferreyra ML, Pezza A, Biarc J, Burlingame AL, Casati P: Plant L10 ribosomal proteins have different roles during development and translation under ultraviolet-B stress. Plant Physiol. 2010, 153: 1878-1894. 10.1104/pp.110.157057.
- 88.
Horiguchi G, Mollá-Morales A, Pérez-Pérez JM, Kojima K, Robles P, Ponce MR, Micol JL, Tsukaya H: Differential contributions of ribosomal protein genes toArabidopsis thalianaleaf development. Plant J. 2011, 65: 724-736. 10.1111/j.1365-313X.2010.04457.x.
- 89.
Rosado A, Sohn EJ, Drakakaki G, Pan S, Swidergal A, Xiong Y, Kang BH, Bressan RA, Raikhel NV: Auxin-mediated ribosomal biogenesis regulates vacuolar trafficking inArabidopsis. Plant Cell. 2010, 22: 143-158. 10.1105/tpc.109.068320.
- 90.
Van Minnebruggen A, Neyt P, De Groeve S, Coussens G, Ponce MR, Micol JL, Van Lijsebettens M: Theang3mutation identified the ribosomal protein geneRPL5Bwith a role in cell expansion during organ growth. Physiol Plant. 2010, 138: 91-101. 10.1111/j.1399-3054.2009.01301.x.
- 91.
Szakonyi D, Byrne ME: Ribosomal protein L27a is required for growth and patterning in Arabidopsis thaliana. Plant J. 2011, 65: 269-281. 10.1111/j.1365-313X.2010.04422.x.
- 92.
Doherty L, Sheen MR, Vlachos A, Choesmel V, O'Donohue MF, Clinton C, Schneider HE, Sieff CA, Newburger PE, Ball SE, Niewiadomska E, Matysiak M, Glader B, Arceci RJ, Farrar JE, Atsidaftos E, Lipton JM, Gleizes PE, Gazda HT: Ribosomal protein genesRPS10andRPS26are commonly mutated in Diamond-Blackfan anemia. Am J Hum Genet. 2010, 86: 222-228. 10.1016/j.ajhg.2009.12.015.
- 93.
Freed EF, Bleichert F, Dutca LM, Baserga SJ: When ribosomes go bad: diseases of ribosome biogenesis. Mol Biosyst. 2010, 6: 481-493. 10.1039/b919670f.
- 94.
Warner JR, McIntosh KB: How common are extraribosomal functions of ribosomal proteins?. Mol Cell. 2009, 34: 3-11. 10.1016/j.molcel.2009.03.006.
- 95.
Deisenroth C, Zhang Y: Ribosome biogenesis surveillance: probing the ribosomal protein-Mdm2-p53 pathway. Oncogene. 2010, 29: 4253-4260. 10.1038/onc.2010.189.
- 96.
Dutt S, Narla A, Lin K, Mullally A, Abayasekara N, Megerdichian C, Wilson FH, Currie T, Khanna-Gupta A, Berliner N, Kutok JL, Ebert BL: Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011, 117: 2567-2576. 10.1182/blood-2010-07-295238.
- 97.
Narla A, Hurst SN, Ebert BL: Ribosome defects in disorders of erythropoiesis. Int J Hematol. 2011, 93: 144-149. 10.1007/s12185-011-0776-0.
- 98.
Abel S, Oeller PW, Theologis A: Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci U S A. 1994, 91: 326-330. 10.1073/pnas.91.1.326.
- 99.
Zenser N, Ellsmore A, Leasure C, Callis J: Auxin modulates the degradation rate of Aux/IAA proteins. Proc Natl Acad Sci U S A. 2001, 98: 11795-11800. 10.1073/pnas.211312798.
- 100.
Nishimura T, Wada T, Yamamoto KT, Okada K: The Arabidopsis STV1 protein, responsible for translation reinitiation, is required for auxin-mediated gynoecium patterning. Plant Cell. 2005, 17: 2940-2953. 10.1105/tpc.105.036533.
- 101.
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O: Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008, 320: 1185-1190. 10.1126/science.1159151.
- 102.
Lanet E, Delannoy E, Sormani R, Floris M, Brodersen P, Crété P, Voinnet O, Robaglia C: Biochemical evidence for translational repression byArabidopsismicroRNAs. Plant Cell. 2009, 21: 1762-1768. 10.1105/tpc.108.063412.
- 103.
Husbands AY, Chitwood DH, Plavskin Y, Timmermans MCP: Signals and prepatterns: new insights into organ polarity in plants. Genes Dev. 2009, 23: 1986-1997. 10.1101/gad.1819909.
- 104.
Wang CY, Chen YQ, Liu Q: Sculpting the meristem: the roles of miRNAs in plant stem cells. Biochem Biophys Res Commun. 2011, 409: 363-366. 10.1016/j.bbrc.2011.04.123.
- 105.
Pinon V, Etchells JP, Rossignol P, Collier SA, Arroyo JM, Martienssen RA, Byrne ME: ThreePIGGYBACKgenes that specifically influence leaf patterning encode ribosomal proteins. Development. 2008, 135: 1315-1324. 10.1242/dev.016469.
- 106.
Braybrook SA Kuhlemeier C:植物如何构建leaves. Plant Cell. 2010, 22: 1006-1018. 10.1105/tpc.110.073924.
- 107.
黄姚Y,凌问,王H, H:核糖体蛋白质promote leaf adaxial identity. Development. 2008, 135: 1325-1334. 10.1242/dev.017913.
- 108.
Mallory AC, Dugas DV, Bartel DP, Bartel B: MicroRNA regulation of NAC-domain targets is required for proper formation and separation of adjacent embryonic, vegetative, and floral organs. Curr Biol. 2004, 14: 1035-1046. 10.1016/j.cub.2004.06.022.
- 109.
Baker CC, Sieber P, Wellmer F, Meyerowitz EM: Theearly extra petals1mutant uncovers a role for microRNA miR164c in regulating petal number in Arabidopsis. Curr Biol. 2005, 15: 303-315. 10.1016/j.cub.2005.02.017.
- 110.
Sieber P, Wellmer F, Gheyselinck J, Riechmann JL, Meyerowitz EM: Redundancy and specialization among plant microRNAs: role of theMIR164family in developmental robustness. Development. 2007, 134: 1051-1060. 10.1242/dev.02817.
- 111.
Byrne ME, Simorowski J, Martienssen RA:ASYMMETRIC LEAVES1revealsknoxgene redundancy inArabidopsis. Development. 2002, 129: 1957-1965.
- 112.
Xu B, Li Z, Zhu Y, Wang H, Ma H, Dong A, Huang H: Arabidopsis genesAS1,AS2, andJAGnegatively regulate boundary-specifying genes to promote sepal and petal development. Plant Physiol. 2008, 146: 566-575.
- 113.
Koyama T, Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M: TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves inArabidopsis. Plant Cell. 2010, 22: 3574-3588. 10.1105/tpc.110.075598.
- 114.
Mordhorst AP, Voerman KJ, Hartog MV, Meijer EA, Van Went J, Koornneef M, de Vries SC: Somatic embryogenesis inArabidopsis thalianais facilitated by mutations in genes repressing meristematic cell divisions. Genetics. 1998, 149: 549-563.
- 115.
Rupp HM, Frank M, Werner T, Strnad M, Schmülling T: Increased steady state mRNA levels of theSTMandKNAT1homeobox genes in cytokinin overproducingArabidopsis thalianaindicate a role for cytokinins in the shoot apical meristem. Plant J. 1999, 18: 557-563. 10.1046/j.1365-313X.1999.00472.x.
- 116.
McIntosh KB, Bonham-Smith PC: Ribosomal protein gene regulation: what about plants?. Can J Bot. 2006, 84: 342-362. 10.1139/b06-014.
- 117.
Hammond MC, Wachter A, Breaker RR: A plant 5S ribosomal RNA mimic regulates alternative splicing of transcription factor IIIA pre-mRNAs. Nat Struct Mol Biol. 2009, 16: 541-549. 10.1038/nsmb.1588.
- 118.
Rosado A, Raikhel NV: Application of the gene dosage balance hypothesis to auxin-related ribosomal mutants in Arabidopsis. Plant Signal Behav. 2010, 5: 450-452. 10.4161/psb.5.4.11341.
- 119.
Stirnberg P, Furner IJ, Leyser HMO: MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 2007, 50: 80-94. 10.1111/j.1365-313X.2007.03032.x.
- 120.
Woo HR, Chung KM, Park JH, Oh SA, Ahn T, Hong SH, Jang SK, Nam HG: ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell. 2001, 13: 1779-1790.
- 121.
Liang J, Zhao L, Challis R, Leyser O: Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum). J Exp Bot. 2010, 61: 3069-3078. 10.1093/jxb/erq133.
- 122.
Ingolia NT, Ghaemmaghami S, Newman JRS, Weissman JS: Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009, 324: 218-223. 10.1126/science.1168978.
- 123.
Leyser HMO, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M:Arabidopsisauxin-resistance geneAXR1encodes a protein related to ubiquitin-activating enzyme E1. Nature. 1993, 364: 161-164. 10.1038/364161a0.
- 124.
Konieczny A, Ausubel FM: A procedure for mappingArabidopsismutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993, 4: 403-410. 10.1046/j.1365-313X.1993.04020403.x.
- 125.
Neff MM, Neff JD, Chory J, Pepper AE: dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications inArabidopsis thalianagenetics. Plant J. 1998, 14: 387-392. 10.1046/j.1365-313X.1998.00124.x.
- 126.
Clough SJ, Bent AF: Floral dip: a simplified method forAgrobacterium-mediated transformation ofArabidopsis thaliana. Plant J. 1998, 16: 735-743. 10.1046/j.1365-313x.1998.00343.x.
- 127.
Gleave美联社:通用的二进制向量系统T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol. 1992, 20: 1203-1207. 10.1007/BF00028910.
Acknowledgements
Mohamed Asrih initially mapped6- - - - - -7to Chromosome 5. We thank Pilar Cubas, Remko Offringa , Tobias Sieberer and the Nottingham Arabidopsis Stock Centre for seeds, and the John Innes Genome Laboratory for providing JAtY TAC clones. The York University horticultural team provided excellent plant care. This work was funded by grants from the BBSRC and the Gatsby foundation. JPL was supported by a scholarship from the State Scholarship Fund of P. R. China, with thanks to the China Scholarship Council (CSC).
Author information
Affiliations
Corresponding author
Additional information
Competing interests
The authors declare no competing interests.
Authors’ contributions
PS isolated the mutant, performed the genetic and phenotypic characterisation, and made theRPS10constructs. JPL, SLK and SW mapped the mutant gene. JPL and PS performed mutant rescue experiments. Excised node assays were carried out by SW. PS and OL wrote the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
Table S1.
Additional file 1:rps10b-1complementation analysis. (PDF 123 KB)
Figure S1.
Additional file 2:rps10b-1does not suppress strigolactone insensitivity ofmax2-1hypocotyls. Relative hypocotyl lengths of light-grown wild-type,rps10b-1,max2-1andrps10b-1 max2-1seedlings after 7 days of growth on vertical sterile agar plates without or with the synthetic strigolactone GR24. Mean hypocotyl lengths (n = 19-28), were normalized to the mean length on control medium for each genotype. Error bars represent the standard error of the ratios. Sterile growth conditions and preparation of GR24 according to [55] except that sucrose was omitted from the growth medium. (PDF 29 KB)
Figure S2.
Additional file 3:rps10b-1does not suppress upregulation of the genes encoding strigolactone biosynthetic enzymes CCD7 (CAROTENOID CLEAVAGE DIOXYGENASE7) and CCD8 inmax2-1 mutant inflorescence stems. RT-PCR analysis of the transcript levels ofCCD7andCCD8in total RNA prepared from basal primary inflorescence stem segments. RT-PCR forUBIQUITIN5(UBQ5) was used as RNA normalization control. (PDF 72 KB)
Figure S3.
Additional file 4: Widespread expression ofRPS10A,RPS10BandRPS10C and lack of tissue-specific variation in their relative contributions to transcript level.RT-PCR analysis of the transcript levels ofRPS10A,RPS10BandRPS10Cin total RNA prepared from different Columbia wild-type Arabidopsis tissues was carried out as described [119].Gene-specific amplification was ensured by reverse priming to divergent 3’-untranslated sequences. RT-PCR forACTIN2was used as RNA normalization control. (PDF 140 KB)
Figure S4.
Additional file 5: rps10b-1 enhances leaf polarity defects of theasymmetric leaves1 (as1)mutant. Theas1-1allele in the Col-1 background (NASC stock N3374) was used in this experiment. Rosette centres of wild type (a),rps10b-1(b) andas1(c) controls and of putative double mutantrps10b-1 as1F2segregants from a cross of the single mutants (d,e). While the oldest leaves of these plants appearedas1-like, younger leaves were trumpet-shaped, or their leaf lamina was strongly reduced (arrows). These segregants bolted normally and produced flowers and seeds. Scale bars: 5 mm in (d) for (a-d) and 1 mm in (e). (PDF 1583 kb) (PDF 2 MB)
作者的起源nal submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open AccessThis article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Stirnberg, P., Liu, JP., Ward, S.et al.Mutation of the cytosolic ribosomal protein-encodingRPS10Bgene affects shoot meristematic function in Arabidopsis.BMC Plant Biol12,160 (2012). https://doi.org/10.1186/1471-2229-12-160
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/1471-2229-12-160
Keywords
- Shoot branching suppressor
- S10e
- Axillary bud
- Leaf polarity
- Lateral organ boundary
- Auxin
- Strigolactone
- CUC
- REV