- Research article
- Open Access
- Published:
基因组学的方法来理解的角色uxin in apple (Malusxdomestica)fruit size control
BMC Plant Biologyvolume12, Article number:7(2012)
Abstract
Background
Auxin is an important phytohormone for fleshy fruit development, having been shown to be involved in the initial signal for fertilisation, fruit size through the control of cell division and cell expansion, and ripening related events. There is considerable knowledge of auxin-related genes, mostly from work in model species. With the apple genome now available, it is possible to carry out genomics studies on auxin-related genes to identify genes that may play roles in specific stages of apple fruit development.
Results
大量的生长素的种子与th相比e fruit cortex were observed in 'Royal Gala' apples, with amounts increasing through fruit development. Injection of exogenous auxin into developing apples at the start of cell expansion caused an increase in cell size. An expression analysis screen of auxin-related genes involved in auxin reception, homeostasis, and transcriptional regulation showed complex patterns of expression in each class of gene. Two mapping populations were phenotyped for fruit size over multiple seasons, and multiple quantitative trait loci (QTLs) were observed. One QTL mapped to a region containing an Auxin Response Factor (ARF106).This gene is expressed during cell division and cell expansion stages, consistent with a potential role in the control of fruit size.
Conclusions
The application of exogenous auxin to apples increased cell expansion, suggesting that endogenous auxin concentrations are at least one of the limiting factors controlling fruit size. The expression analysis ofARF106linked to a strong QTL for fruit weight suggests that the auxin signal regulating fruit size could partially be modulated through the function of this gene. One class of gene (“大酒店”3) removes free auxin by conjugation to amino acids. The lower expression of these“大酒店”3genes during rapid fruit expansion is consistent with the apple maximising auxin concentrations at this point.
Background
The hormonal control of fruit growth and development has been well established across many different plants. One hormone, auxin, has been shown to control the initial growth and expansion of tissues following fertilisation [1,2] and inhibit ripening. Early work with strawberry and other fruits proposed a mechanism whereby auxin produced by the developing seed regulated fruit growth by controlling firstly cell division and secondly cell expansion. As the seeds subsequently mature, auxin concentrations drop, acting as a signal for ripening to proceed. Supporting this mechanism is the observation that applied auxins can induce parthenocarpy in fruits such as tomato [3], fruit size in peach [4], cell enlargement in cherry [5] and delay ripening in strawberry [1].Developmental regulation by the principal auxin in higher plants, IAA (Indole Acetic Acid), is achieved through the coordination of complex processes: auxin metabolism (involving biosynthesis, conjugation and catabolism), auxin transport (long distance and polarised auxin transport) and auxin signalling (perception, transduction and response). The balance of synthesis, breakdown, conjugation and transport is tightly regulated, leading to auxin homeostasis [6].
De novoauxin synthesis in plants results from multiple pathways dependent or independent of tryptophan [7,8].IAA can be conjugated to amino acids, sugars and methyl esters. Enzymes that conjugate IAA to amino acids are encoded by members of the group II of the“大酒店”3(Gretchen Hagen 3) family of auxin-induced genes [9].Very little is known about the role of“大酒店”3genes during fruit development. However, it has recently been shown in grape that“大酒店”3.1plays a role in the formation of IAA-Asp late during development, coinciding with the onset of ripening [10].Release of IAA from IAA conjugates is achieved by hydrolytic cleavage [11].Auxin transport from sites of synthesis to target cells is complex and highly regulated, playing a crucial role in both establishing and changing homeostasis. Auxin is transported both passively through the vasculature and actively through transporters [12].The most characterised auxin transport family is the efflux carrier PIN family. PIN proteins are vital for normal plant development. Mutations in thePIN1gene lead to pin-like organs with no development of flower parts inArabidopsis thaliana(Arabidopsis) [13] and members of thePINfamily are highly expressed early during tomato fruit development, suggesting a role during fruit set [14].
The current model for auxin perception and signalling involves two types of receptors [15,16]: the Auxin Binding Protein 1 (ABP1), located at the plasma membrane, and the Transport Inhibitor 1/Auxin signalling F-Box family (TIR1/AFB), a set of nuclear receptors [17–19].ABP1 is involved in very early auxin responses leading, for example, to the modification of ion fluxes [20].ABP1 has been shown to be essential for plant life (a mutation inABP1inArabidopsisis lethal) and is important for both cell division and cell expansion [21–23].However, the details of the pathway going through ABP1 are poorly understood. In tomato, thediageotropica(dgt) mutant displays many auxin-related developmental defects and fruit development is dramatically altered, with a reduced fruit size [24].DGTencodes a cyclophilin, known to act as signalling intermediate, and was shown to use ABP1 as an extracellular receptor for auxin-dependent cell growth response [25].The signalling pathway involving TIR1 is now well characterised and explains most of auxin-regulated gene expression [16].The three families of early auxin responsive genes,Aux/IAA, GH3andSAUR (Small Auxin Up Regulated), contain a binding motif to the ARF transcription factor (Auxin Response Factor). At low auxin concentrations, a heterodimer of an ARF and an Aux/IAA protein represses transcription. At higher auxin concentration, auxin will bind to TIR1/AFB, an F-box protein that is part of an SCF complex (Skp1/Cullin/F-box), and triggers the degradation of the repressor Aux/IAA through the 26S proteasome. This will ultimately release the ARF transcription factor to modulate expression of early auxin response genes. In fleshy fruits, most of our knowledge involving the ARF-Aux/IAA complex during fruit development comes from studies in tomato.SlARF7is expressed in placental and ovule tissues and down-regulated soon after pollination. Silencing of theSlARF7gene leads to parthenocarpic fruit development, showing thatSlARF7functions as a negative regulator of fruit set [26].Similarly, silencing of theSlIAA9gene expression also confers parthenocarpy [27].SlARF4(also known as DR12) seems to play a role later in fruit development, as its expression increases throughout tomato fruit development, with the highest levels in early red-stage fruit. Down-regulation ofSlARF4leads to pleiotropic phenotypes including dark-green immature fruit, enhanced firmness and unusual cell division in the fruit pericarp, which is thicker than in wild-type (WT) fruits [28,29].
While many fleshy fruits are carpel derived, the fruit fromMalusxdomestica(apple) is unusual, as it is derived from the hypanthium, a tube of fused sepals, petals and anther derived tissue. However, like other fruits, apple development can be separated into periods of cell division, cell expansion, maturity, and ripening [30].While there have been a few studies on auxin content in apple [31,32], there is little research reported on the role of auxin in apple fruit development at the molecular level. There are a large number of different cultivars of apples showing a range of different flowering times, maturity times and times to ripen. One cultivar, 'Royal Gala', is a naturally occurring sport of the 'Gala' cultivar. It is a mid-season apple, and its growth and development has been well characterised. 'Royal Gala' has been the subject of a number of genomics studies, including a large-scale expressed sequencing tag (EST) sequencing project [33] and a microarray study of the fruit development [30] and fruit ripening [34].It is readily transformable, with transgenic apples forACO1suppression [34,35],MYB10overexpression [36], andPOLYGALACTURONASE 1[37] being reported. Recently a parent of 'Royal Gala', 'Golden Delicious', has had its genome sequenced [38].
Here we have investigated the role of auxin on apple fruit development and assessed the expression of genes involved in homeostasis, transport and signalling of auxin. The location of auxin-related genes in the genome sequence of apple was compared with QTLs for fruit weight, which is linked to fruit size.
Results
Changes in auxin content over fruit development
Apple fruit have previously been given four distinct developmental stages following pollination, consisting of Stage 1 (cell division), Stage 2 (cell expansion), Stage 3 (fruit maturity) and Stage 4 (ripening) [30].In 'Royal Gala' apples, Stage 1 takes 0-25 days after full bloom (DAFB), rapid growth (Stage 2) covers 20-60 DAFB, after which the fruit continue to grow at a slower rate as the fruit matures (Stage 3), with the ripening process starting around 132 DAFB (Stage 4), with tree-ripe eating apples at 146 DAFB [30].
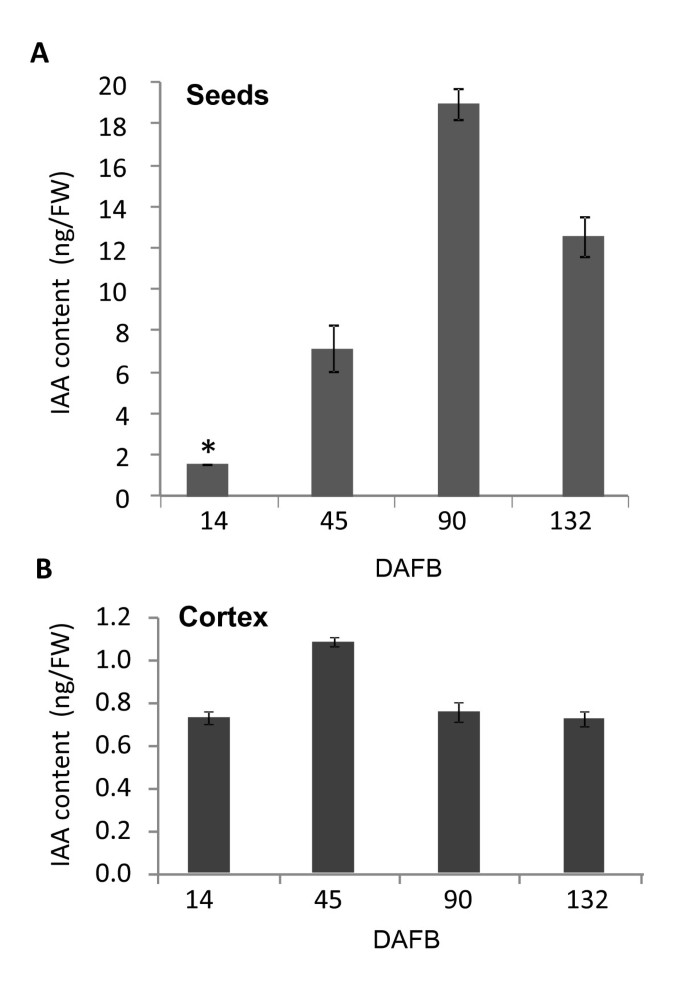
To investigate the role of auxin during fruit development, the free IAA amounts were measured at representative times (14, 45, 90 and 132 DAFB) during the different fruit development stages in 'Royal Gala' fruit cortex and seed. IAA concentrations in the seed showed a large increase during fruit development, reaching a maximum concentration of 19 ng/g fresh weight (FW) (Figure1A).The IAA concentrations in the fruit cortex were considerably lower than in the seed, ranging from 0.6 to 1 ng/g FW, with a significant increase during cell expansion (Figure1B).This is consistent with the literature, showing high auxin concentrations in the seeds of tomato and strawberry, compared with those in the fruits [2,39].
苹果免费生长素含量的组织. Changes in free IAA (Indole Acetic Acid) content (ng/g fresh weight; FW) in the seed (A) and cortex (B) of developing 'Royal Gala' apples over fruit development (DAFB: Days After Full Bloom). Measurements were performed on 4 and 2 biological replicates for cortex and seed samples respectively. * indicates seed sample for which only one extraction was possible. Error bars represent standard error of the mean.
Effect of auxin treatment on apple fruit growth
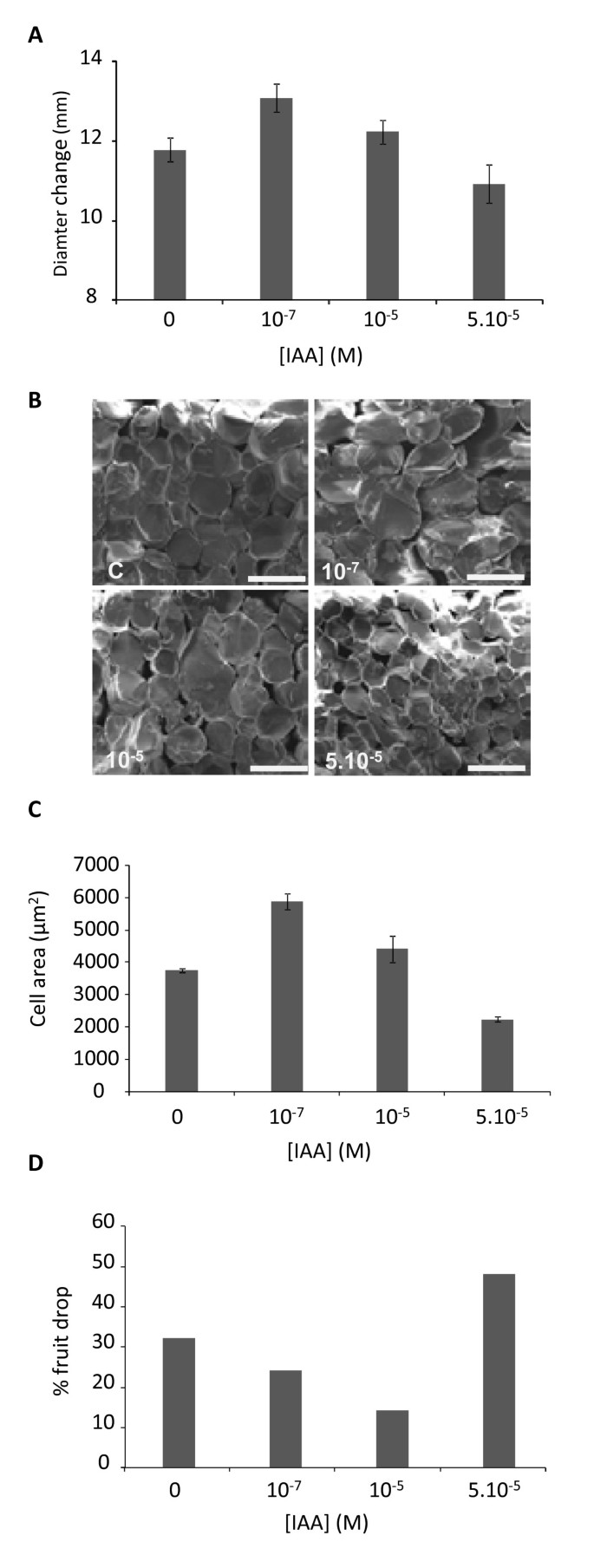
As there was an increase in auxin in the cortex tissue during Stage 2 (the rapid cell expansion phase), we assessed the effect of injecting three auxin concentrations within the physiologically active range for auxins (10-6M) into fruit at the beginning of Stage 2 (30 DAFB). The growth of each apple was assessed by recording the diameter of the fruit at injection and after two weeks of subsequent growth. During the two-week period, all fruit showed an increase in fruit size (Figure2A).The two lowest auxin concentrations (10-7M and 10-5M) caused a significant increase in fruit diameter compared with the control (Figure2A), while the highest concentration (5.10-5M) appeared to inhibit fruit growth. The increased fruit growth observed with the 10-7M and 10-5M treatments corresponded to a greater increase in the cell size (Figure2B,C) compared with that of control apples. During the early stages of apple development, there is a natural self-thinning event. Apples typically have 5 florets per cluster, which for commercial purposes are thinned to 1-2 fruit per cluster, depending on the localised fruit load. Two to three apples were chosen per cluster for injection and the rest were hand thinned. During the two-week treatment, the control showed a 32% fruit drop. When injected with auxin at 10-7M and 10-5M, a higher degree of fruit retention was observed, with only 14% fruit dropped in the 10-5M treatment. Additional auxin promoted fruit drop, with 48% fruit abscised from the 5.10-5M concentration (Figure2D).
The effect of auxin on cell expansion. Indole Acetic Acid (IAA) was injected at different concentrations (10-7M, 10-5M and 5.10-5米)通过“皇家联欢晚会”的花萼苹果30天s after full bloom. Two weeks following injection, diameter increase was measured (A), Cryo-Scanning Electron Microscopy photographs of five representative fruits were taken (scale bar: 100 μm) (B), average cell area was calculated (C) and the percentage of abscised fruit was determined (D). Fifty fruit were injected per concentration. Error bars represent standard error of the mean.
Genomic screening of auxin-related genes in the apple genome
The apple draft genome [38] was screened for six classes of auxin-related genes. These included the receptor-like genesABP1andTIR1/AFB, the transcriptional control genesARFandAux/IAA, and the auxin homeostasis genes, thePINgenes and“大酒店”3-like genes. All six classes of genes searched were well represented in the apple genome (Table1, full list in Additional file1).苹果基因组已经被证明有经历ne a genome duplication, so the numbers of genes were compared with the numbers of auxin-related genes in the recently published woodland strawberry genome [40] (a related diploid Rosaceae species) (Summarised in Table1, and a full list in Additional file2).In each gene family, there are approximately twice the numbers of auxin-related genes in apple than in strawberry.
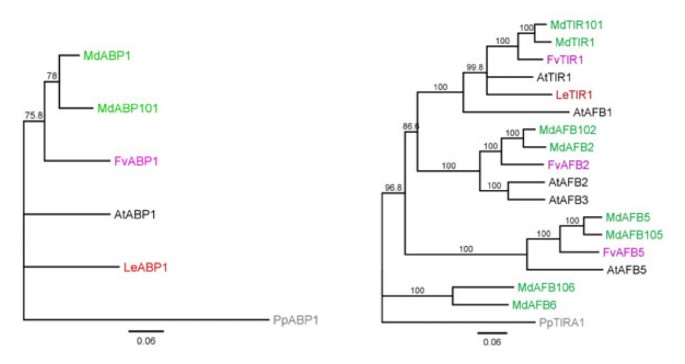
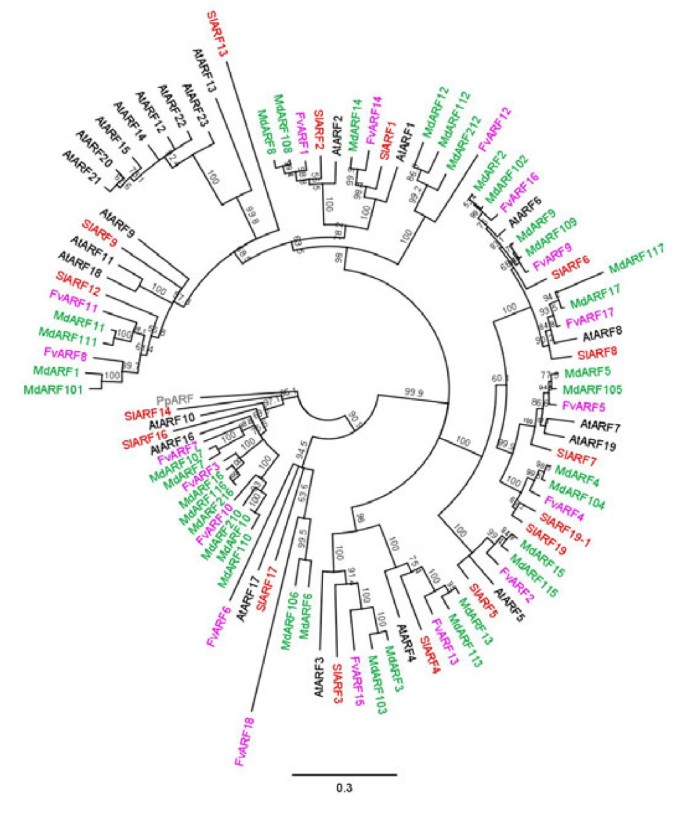
A phylogenetic analysis of the six classes of genes using the predicted protein sequence from apple, strawberry, tomato andArabidopsiswas performed for each class of genes (Figures3,4Additional files2,3).In each of the phylogenetic clusters, the majority of the apple genes were contained in subclades consisting of a single strawberry gene. These subclades typically had two apple duplicated genes for each strawberry gene, with the occasional subclade showing a single apple gene, or three apple genes per strawberry gene. This 2:1 ratio of genes was robustly observed in theABP1, TIR1/AFBandARFclass of genes, with Aux/IAA, GH3andPINshowing a less robust pattern with some strawberry and apple genes showing no corresponding related sequences. The predicted location of the two apple duplicates genes were often found on homeologous chromosomes identified in the apple genome [38] (chromosomal locations are given in Additional file1), with duplicated genes found on non-homeologous chromosomes occurring 18% of the time. Because of this tight phylogenetic relationship between the strawberry genes and two apple genes, when possible both apple homologues were named after the already annotated strawberry genes, for exampleARF1of strawberry clustered with genes in apple that were labelledARF1andARF101. This nomenclature was not followed if the gene had previously been published or released in GenBank. With these genes, the existing name was kept (Additional file1).
Phylogenetic tree of the auxin receptors. (A) ABP1 class of receptors, (B) TIR1/AFB class of receptors. Maximum alignable protein sequences of the auxin receptors from apple (green), strawberry (lilac),Arabidopsis(black) and tomato (red) were aligned using MUSCLE and phylogenetic trees were built using neighbour joining. Bootstraps of 1000 iterations are given.At: Arabidopsis thaliana, Fv: Fragaria vesca, Md: Malusxdomestica, Pp: Physcomitrella patens, Sl: Solanum lycopersicum
Phylogenetic tree of the auxin response factors. The DNA binding domains of the auxin response factors (ARF) from apple (green), strawberry (lilac),Arabidopsis(black) and tomato (red) were aligned using MUSCLE and phylogenetic trees were built using neighbour joining. Bootstraps of 1000 iterations are given.At: Arabidopsis thaliana, Fv: Fragaria vesca, Md: Malusxdomestica, Pp: Physcomitrella patens, Sl: Solanum lycopersicum
While the gene families from the two Rosaceae species, strawberry and apple, were tightly aligned with each other, there was considerable divergence from theArabidopsisgenes. For example, there is evidence of clade expansion in theARFgenes inArabidopsisincluding theAtARFs 12, 14, 15, 20, 21, 22, 23(Figure4).TheTIR1-like proteins also suggests small family expansion inArabidopsis(Figure3).Small subclades containing only proteins from strawberry, apple and tomato were also observed.
Expression analysis of auxin-related genes during apple fruit development
Gene expression for each of the auxin-related genes was screened using quantitative polymerase chain reaction (PCR) across flowering and at time points representing the four stages of apple fruit development (0, 14, 45, 90, 132 DAFB) (Additional file4).Some of the homeologous genes showed very little sequence divergence at the DNA level, making it hard to select optimal qPCR primers that were specific for each gene in the homeologous pairs. Of the 108 genes tested, 25 primer pairs were predicted to be unable to differentiate between the homeologues (Additional file4).In these instances, the expression patterns are given with both names (Figure5) or marked with an asterisk (Figures6,7).
Expression analysis of auxin receptor class of genes. Expression analysis (by qPCR) for the auxin receptorsABP1andTIR1/AFB类的基因通过五个阶段的fr所示uit development (0: fruit set, 1: cell division, 2: cell expansion, 3: fruit maturation and 4: fruit ripening) represented by fruit harvested at 0, 14, 45, 90, 132 Days After Full Bloom (DAFB). Where the primers were unable to distinguish between the homeologous genes, both gene names are given. Expression is relative to actin, with error bars representing standard error of the mean (n = 4)
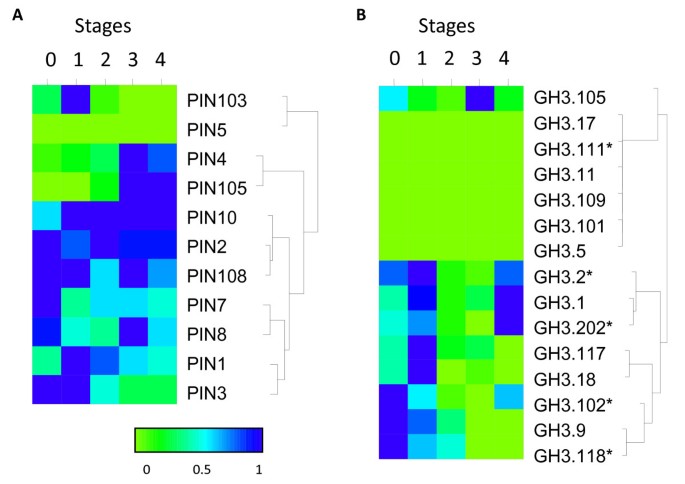
Expression analysis of auxin homeostasis genes. Expression analysis (by qPCR) of auxin-conjugating enzyme (“大酒店”3) and transport genes (PIN) over fruit development are grouped by hierarchical clustering, normalised to maximum expression of each gene. Five stages of fruit development (0: fruit set, 1: cell division, 2: cell expansion, 3: fruit maturation and 4: fruit ripening) represented by fruit harvested at 0, 14, 45, 90, 132 Days After Full Bloom (DAFB). Asterisks represent primer pairs unable to distinguish between homeologous genes.
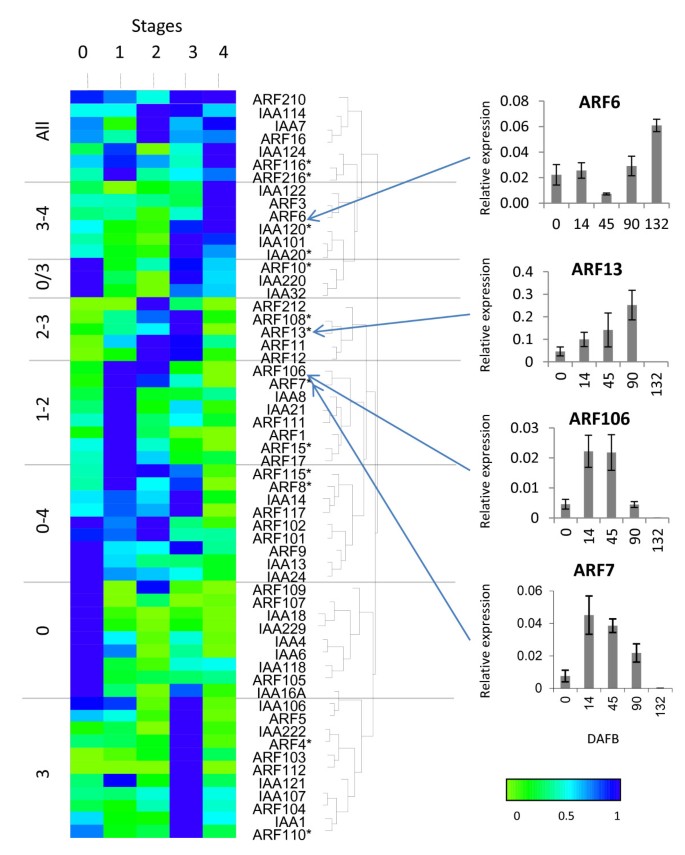
Expression analysis of auxin transcriptional regulators. Expression of the transcriptional regulatorsARFandAux/IAAclass of genes are clustered according to expression patterns over fruit development, grouped by hierarchical clustering, normalised to maximum expression of each gene. Five stages of fruit development (0: fruit set, 1: cell division, 2: cell expansion, 3: fruit maturation and 4: fruit ripening) are represented by fruit harvested at 0, 14, 45, 90, 132 Days After Full Bloom (DAFB). Asterisks represent primer pairs unable to distinguish between homeologous genes. The expression patterns of selected genes within the cluster are also presented. Expression is relative to actin, with error bars representing 4 replicates.
The genes were divided into three main functional groups for analysis: receptors (Figure5), homeostasis (Figure6) and response (Figure7).When it was possible to differentiate the homeologous genes, the expression patterns within each pair were compared with each other. There were a considerable number of homeologous genes with different expression patterns and also instances where one of the homeologous genes was apparently switched off, for exampleABP1andABP101(Figure5).This may be because of the quality of the primer, or actual lack of expression. To address this concern, we examined the EST libraries [33] for ESTs corresponding to the 10 receptor-like genes, and used diagnostic DNA polymorphisms to identify each of the homeologues. From these sequence data, it appears thatABP1is transcribed andABP101is not (confirming the qPCR data). For theTIR1/AFBclass there was a similar pattern, withTIR1, AFB102, andAFB106having ESTs, whileTIR101, AFB2, andAFB6were not represented, suggesting that the transcription of these genes is suppressed. For the apparent lowly expressedAFB5andAFB105there were no ESTs with which to compare. Both theABP1andTIR1class of receptor genes were expressed constitutively through fruit development. In theTIR1/AFBfamily,AFB102showed a Stage 1 and 2 predominant expression, whileAFB106showed a high degree of expression at Stage 4 fruit development (Figure5).
PIN103showed the highest expression only during the cell division (Stage 1), whilePIN4andPIN105showed predominant expression as the fruit progressed into maturity and ripening (Stages 3-4) (Figure6A).Half the“大酒店”3conjugating proteins showed high expression levels early in fruit development - cell division (Stages 0 and 1), while all were suppressed during Stages 2-3 (cell expansion), with a number increasing again as the fruit ripened (Figure6B).
Many of theARF/Aux/IAAfamily of genes had discrete expression patterns for a single stage of fruit development (Figure7).Half the differentially expressed genes were predominantly expressed during the early stages of fruit development, with a high proportion of Aux/IAAgenes being highly expressed at full bloom. As auxin has been shown to be central to the regulation of fruit size, which is determined during cell division and cell expansion, we were particularly interested in profiles that had high expression in Stages 1 and 2. In this cluster, fourARF和两个Aux/IAAhad highest expression during Stage 1. WhileARF212had peak expression at Stage 2, it had a very low expression level. Only two genes had high expression during both these two stages (ARF7andARF106) (Figure7), suggesting these two genes may play a role in the control cell division and/or expansion affecting final fruit size.
QTL mapping for fruit size
To assess regions controlling fruit size in apples, two mapping populations were assessed for fruit weight, as a surrogate for fruit size, over a number of successive years. Fruit weight was phenotyped for a total of 572 and 123 genotypes from 'Royal Gala' × 'Braeburn' (RGxBB) and 'Starkrimson' × 'Granny Smith' (STKxGS) mapping populations. Measurements were made over 2 and 5 years of production, and two and one sites, respectively. Analysis of variance showed that the genotypic (G) and the year (Y) effects for fruit weight were highly significant for each population (Pvalues < 2.2e-16). For the RGxBB population, the effects of the growing environment (E;Pvalue < 2.2e-16) and its interaction with the genotype (GxE;Pvalue < 2.2e-16) were highly significant. Best Linear Unbiased Predictors (BLUPs) independent of year and environment were extracted for each genotype for both studied populations and were used as phenotypic data for QTL detection. Six QTL regions were identified for fruit weight using the RGxBB and STKxGS genetic maps on Linkage Group (LG) 5, 8, 11, 15, 16 and 17 (Table2, Additional file5).Two QTL regions were conserved across both segregating populations on LG 8 and LG 15. The explained genetic variability (R2) for each of the QTLs ranged between 3.9% for a 'Royal Gala' QTL on LG 15 to 17.3% for a 'Granny Smith' QTL on LG 8. The globalR2were higher in the STKxGS segregating population (53.9%) than in the RGxBB (18.2%). The QTLs detected in the RGxBB segregating population were not involved in any epistasic effect, whereas the three fruit weight QTLs detected in the STKxGS population on LG 8, 15 and 17 were involved in an epistatic effect.
Characteristics of the QTLs detected on separated parental genetic maps, 'Royal Gala', 'Braeburn' (RGxBB), 'Starkrimson' and 'Granny Smith' (STKxGS) map by Multiple QTL Mapping(MQM) mapping for fruit weight. QTL detection was carried out using Best Linear Unbiased Predictor (BLUP) as phenotypic data. Different BLUP values were extracted to represent genetic potential for each genotype (Fruit Weight), fruit weight in a given year calculated from the Genotype × Year (GxY) interaction, and fruit weight in a given environment calculated from the Genotype × Environment (GxE) interaction. For the RGxBB population, BLUP values were extracted for the genotype (Fruit Weight), the interaction G × Y (Fruit Weight_2009 and Fruit Weight_2010), and the interaction G × E (Fruit Weight_Hawke's Bay and Fruit Weight_Nelson). For the STKxGS population, BLUP values were extracted for the genotype (Fruit Weight) and for each year (Fruit Weight_2006, Fruit Weight_2007, Fruit Weight_2008, Fruit Weight_2009 and Fruit Weight_2010). For each QTL, the table displays the marker used as a co-factor for MQM analysis, the LOD score, and the percentage of genetic variation explained by each single QTL (R2).When several QTLs were detected for the same trait, the globalR2(the proportion of variation explained by the QTLs) and the interactions between QTLs are indicated.
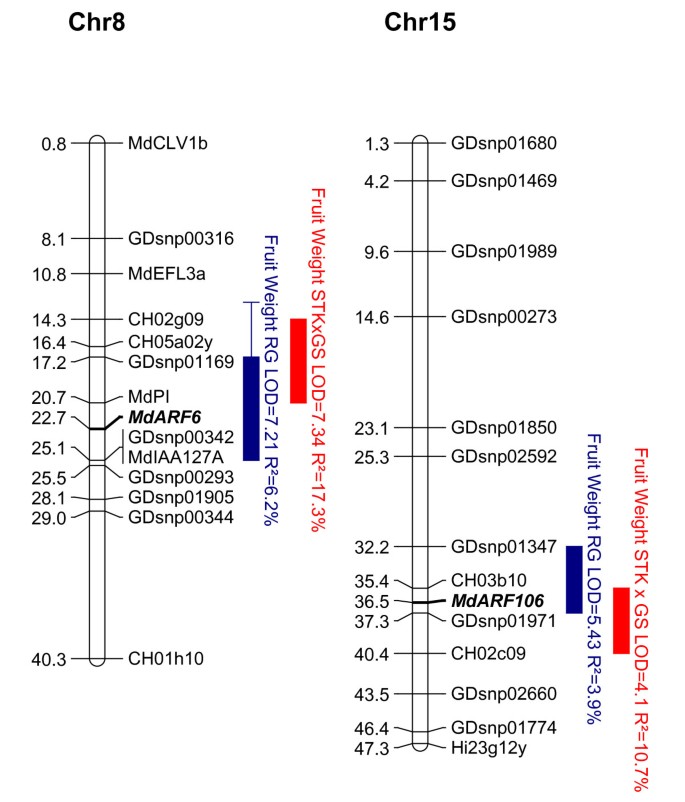
The six QTL regions were compared with thein silicolocations of auxin genes that showed predominant expression during Stages 1 and 2.ARF106is located on LG 15 and could account for the fruit weight QTL on this linkage group. The genetic marker developed from the sequence ofARF106had the highest LOD score (logarithm of odds) for the fruit weight QTL, in both the 'Royal Gala' and 'Starkrimson' × 'Granny Smith' genetic maps (Table2and Figure8).The QTL interval spanned an overlapping area between both STKxGS and RGxBB QTLs, of 1.92 Mb, from markers CH03b10 (35.397 Mb) to GDsnp01971 (37.346 Mb). Within this region, in addition toARF106(MDP0000232116), 132 other predicted gene models were found (Additional file6).While some of these genes showed homology toArabidopsisgenes that have annotated gene ontology, which may control fruit size, such as involvement in cell division, cell cycle and signal transduction (Additional file6), these were not studied further as they were outside the scope of this project.
Quantitative Trait Loci (QTL) for apple fruit size. Physical positions (Mb) of the Quantitative Trait Loci (QTLs) detected on the parental 'Royal Gala' genetic map (RG, blue) and on the consensus 'Starkrimson' × 'Granny Smith' (STK × GS, red). QTLs are represented by boxes, in which length represents the LOD-1 confidence interval and extended lines represent the LOD-2 confidence interval. Mapped candidate genesARF6andARF106are in bold underlined.
The LG 8 and 15 QTLs were mapped in duplicated regions of the apple genome because of whole genome duplication [38].The homeologous gene toARF106isARF6. While expressed later in fruit development, there is still the possibility thatARF6retains some residual fruit size control. The genetic marker developed fromARF6mapped to the fruit size QTL but was not the strongest marker in this region (Table2and Figure8).
Discussion
With the availability of the whole genome sequence for apple [38], there is now the possibility of identifying the complete gene family for different classes of genes. This powerful resource has been used to identify individual auxin gene classes (mostlyARFandAux/IAA) in a number of plants includingArabidopsis[41], poplar [42], maize [43,44] and tomato [45].The auxin signal has been implicated in many components of fruit growth and development, including determination of fruit size through cell expansion, as well as the control of fruit ripening, and the regulation of fruit drop [46].Here we have presented a genomics screen in apple of different auxin-related genes, covering perception, homeostasis and transcriptional regulation, and their relative expression patterns over fruit development. These expression data provide the ground work for further studies in the role of auxin on apple fruit growth and development.
Fruit size determination
There are a number of factors that regulate fruit size in apples, those that are controlled by the genetic make-up of the apple (size potential), and those related to the environment in which the apple develops. The environmental control of size is determined by both the effects of orchard husbandry and irrigation and local effects in the apple tree, such as fertilisation success, and localised crop load. We demonstrated that in the RGxBB segregating population, the effect of the year, the environment and its interaction with the genotype were highly significant. Furthermore, a significant difference was observed in the global phenotypic variation explained by the QTLs for fruit size detected in the two segregating populations. The larger explained phenotypic variation for the STKxGS population can be explained by the fact this population was grafted on less dwarfing rootstock ('Pajam') than the RGxBB ('M.9'). In addition, the crop load for the RGxBB was regulated, while the STKxGS was not. While the environment can cause significant amounts of variation, the genetic size potential of the fruit is a major determinant of fruit size. This work and others have shown that size potential is a complex multi-loci trait in apples [47和番茄等水果48,49].在苹果果实大小的控制nked to both the number of cell division steps that occur directly following pollination and to subsequent cell expansion [50].Apples have been shown to have a range of different cell sizes across different cultivars [51], and increasing cell number and size through endo-reduplication causes a 38% increase in fruit weight [52].Our work suggests that auxin signals through this cell division/cell expansion phase may be modulated by an ARF gene (ARF106) that is up-regulated during these developmental time points and co-locates with a stable QTL for fruit weight.ARF106is most closely linked to theAtARF17, which is microRNA controlled and when over-expressed using a microRNA-resistant form, gives a pleotropic phenotype [53] including excess tissue growth in leaves. The corresponding ARF in tomatoSlARF17is expressed highly at fruit ripening (namedSlARF13in [45], see Additional file2).
The balance of auxin is critical for fruit expansion. This auxin signalling is complex, as not only can the presence of auxin elicit a developmental response, but different concentrations can cause different responses [54].Here we found that injecting different concentrations of auxin could cause an increased cell expansion, decreased fruit growth, and ultimately fruit drop [55].The increased cell growth with lower amounts of injected auxin suggests that fruit growth is at least in part limited by auxin concentration, as application of more can enhance it, which is consistent with the observations of Percy and collaborators [56].
Extensive molecular research on auxin inArabidopsishas now identified a number of key genes involved in the regulation of auxin content of plant tissue, and the method by which the auxin signal is converted into a developmental change. Part of the complexity of the auxin signal transduction can be explained by the regulation of response genes: by developmentally regulating both the signal transduction transcription factors (ARF) and the modulators of transcription (Aux/IAA), a complex network of regulation can be achieved. This complexity can explain how the same signalling molecule can relay different signals at different times during development [6].
Auxin-related processes in fruit set
While the molecular control of auxin is best understood in the model plantArabidopsis, which bears dry dehiscent fruit, there has been a substantial body of work in fleshy fruit species. Surprisingly, for many of the ARF proteins, the tomato orthologue (Asterid) is more closely related to the apple gene than theArabidopsisorthologue (a Rosid, like apple). The ARF proteins, which are more similar to those in tomato (MdARF1, 101, 11, 111, 8, 108, 4, 104, 13 113, 3, 103) are all expressed at a specific stage in fruit development (Figure7).这可能表明一个强大的进化压力for conservation within fleshy fruit species. This conservation is also observed in the expression patterns presented in [45], with genes such asMdARF113/SlARF4, MdARF4/SlARF19expressed at similar times in fruit development. The phylogenetic discrepancy is also observed in the IAA cluster (Additional File3); however, there are currently no published genomics studies of IAA genes in tomato to make this comparison.
Key auxin-related genes such asAtARF8(FWF) inArabidopsisandSlARF7in tomato have been associated with fruit set [26,57].Mutations inAtARF8and down-regulation ofSlARF7cause parthenocarpic fruit inArabidopsisand tomato respectively, indicating thatAtARF8is functionally equivalent toSlARF7, while it is not the closest homologue (Figure4) [58].Down-regulation ofSlIAA9also leads to development of parthenocarpy and it has been hypothesised that it could work in the same pathway asSlARF7[59].SlARF7clusters withARF5andARF105, AtARF8clusters withARF17andARF117, whileSlIAA9clusters withIAA8, IAA27AandIAA127A(Figure4, Additional file3).Of these apple genes,ARF105has highest expression at full bloom, likeSlARF7[26,45] (namedSlARF9in this later study), suggesting that this may play a similar role, whileARF5is undetectable.
Auxin and ripening
The role of auxin in the regulation of ripening in strawberry has been well established after it was found that application of auxins through the peduncle caused a significant delay in ripening [1].This link suggesting that auxin is a negative regulator of ripening was further enforced by a study over-expressing a pepper“大酒店”3-related gene in tomato [60].In this study, theCcGH3over-expressing lines matured earlier than untransformed lines and ripened earlier with the addition of exogenous ethylene. The closest homologue from of thisCcGH3in apple is“大酒店”3.1, which also shows a large increase in expression around fruit maturity (Figure6B).Interestingly, this gene is also expressed during the cell division stage. As well as the“大酒店”3-related genes, there is also a cluster of auxin signal transduction genes that are up-regulated at Stage 4 (Figure7), suggesting that an auxin signal can still be transduced at this stage. This is consistent with auxin playing a role in fruit maturation and ripening that is well known in non-climacteric fruits [1,10] and beginning to be established in climacteric fruit [61].
Another gene in tomatoSlIAA3is induced by both ethylene and auxin at fruit maturation [62].SlIAA3clusters withIAA6andIAA106, neither of which is expressed at the ripening stage. However, 6ARF/Aux/IAAgenes are highly expressed at ripening, suggesting a similar role in apple. Down-regulation of another tomato auxin response factor,SlARF4(also referred initially as DR12) [28,29], leads to unripe fruit that are firmer because of a perturbed pectin metabolism, and the fruit display an unusual cell division pattern in the pericarp.SlARF4clusters withARF13andARF113, of whichARF13is expressed during cell expansion and maturation. This differs slightly fromSlARF4, which shows increasing expression during tomato fruit development, with the highest in ethylene-producing fruits.
Conclusions
This work has provided a genomics study of auxin regulation in apples, with many auxin-related genes changing through fruit development. The complexity of expression patterns of these genes suggests a complex role of auxin regulation in apple fruit development. Exogenously applied auxin during the end of cell division/early cell expansion phase can increase fruit size, showing that auxin is at least in part one of the limiting factors controlling cell expansion. The role of one auxin response gene,ARF106, which maps to a size-related QTL, needs to be further investigated to determine if this gene plays a role in apple fruit size regulation.
Methods
Selection of genes in the apple genome
Auxin-related genes were selected by using BLASTP search of knownArabidopsisauxin-related genes against predicted apple protein sequences within the 'Golden Delicious' whole genome sequence [38].The predicted MDP numbers were mapped to chromosome location using the appleMalusxdomesticaGBrowse from the Genome Database for Rosaceae (http://www.rosaceae.org/gb/gbrowse/malus_x_domestica/) (Additional file1).The protein sequences were compared with strawberry andArabidopsisgenes based on phylogeny. For each gene family, the longest highly conserved alignable protein sequence regions were used as detailed below: ABP1: the whole protein without the leader peptide. TIR1/AFB: The F-box domain and the leucine rich repeat domains. ARFs: The DNA binding domain. AUX/IAA domains I-IV. PINs: Transmembrane domains. GH3: The whole protein. Apple auxin-related genes were named firstly on published GenBank accessions and secondly on the nearest strawberry-named gene in the strawberry predicted protein sequences V2. Alignments and phylogenetic trees were generated using the Geneious Pro™ 5.4 (Biomatters). Multiple alignments were performed using MUSCLE and phylogenetic trees were built using neighbour joining with 1000 bootstraps. Sequences from the mossPhyscomitrella patenswere used to root the trees. Accession numbers of proteins fromArabidopsis, strawberry and tomato are given in Additional file2.
Auxin injection in apples and assessment
Developing apple fruit were injected with different concentrations of IAA (Sigma-Aldrich, UK), dissolved in a 0.1% ethanol solution, 30 days after full bloom. Control apples were injected with a 0.1% ethanol solution. For each treatment, 100 μL were injected through the calyx using a syringe, into a minimum of 50 apple fruit; the apple diameter was measured on its equator, and the fruit tagged. Fifteen days after injection, the apples were harvested and the apple diameter was re-measured to establish the growth rate. For each treatment, the cortex tissue adjacent to the calyx from five representative apples was assessed using freeze fracture scanning electron microscopy (SEM). CryoSEM was performed using a Polaron PP2000 Cryo Transfer system (Quorum Technologies, Ringmer UK) attached to an FEI Quanta250 Scanning Electron Microscope (FEI Hillsboro OR). Blocks of apple tissue about 4 × 6 × 2 mm were placed in aluminium sample holders, held in brass transfer shuttles, using a mixture of colloidal graphite and OCT™ compound (Sakursa Finetek, Zoeterwoude, NL) as adhesive, so that a portion of the apple protruded from the surface. These were frozen in liquid nitrogen slush. Samples were transferred under vacuum to the PP2000 preparation stage, which was held at -150°C and the apple tissue fractured using a cooled metal blade or probe. The fractured surface was sputter-coated with gold/palladium (60 sec) and transferred to the SEM for observation on a stage cooled to -150°C using an accelerating voltage of 15 kV. Cell size was measured by counting the number of whole cells in the fracture window from each of the five treatment samples.
Auxin content measurement
IAA was extracted from 'Royal Gala' cortex (4 replicates) and seed (2 replicates) at different times during fruit development. Tissue samples were homogenised and IAA was extracted with 80% (v/v) methanol containing 250 mg l-1butylated hydroxytoluene. 10 ng of [13C6]IAA, internal standard was added to extracts and left at 4°C for 24 h. The extracts were then filtered through Whatman no. 1 filter paper. Samples were reduced in volume to less than 1 mL under vacuum at 35°C and an aliquot was loaded onto a Sep-Pak C18 cartridge in 0.4% acetic acid. IAA was eluted with 50% methanol in 0.4% acetic acid. The eluate was dried and taken up in 1% acetic acid. Samples were then analysed using a Waters Acquity H-series UPLC coupled to a Waters Xevo triple quadrupole mass spectrometer. A Waters Acquity UPLC BEH C18 column (2.1 × 100 mm × 1.7 μm particles) was utilised. The solvents were 1% acetic acid in water (Solvent A) and acetonitrile (Solvent B) at a flow rate of 0.25 mL/min, with a linear gradient from 80% A:20% B to 50% A:50% B at 4.5 mins, followed by re-equilibration to starting conditions for 3 mins. Five μL of each sample was injected. The mass spectrometer was operated in positive ion electrospray mode with a needle voltage of 2.4 KV, and selected reaction monitoring was used to detect IAA and13C6IAA. The ion source temperature was 150°C, the desolvation gas was nitrogen at 1000 L/h, the cone gas flow was 50 L/h and the desolvation temperature was 300°C. The MS/MS transitions monitored were m/z 176.2 to 130.1 for IAA and 182.2 to 136.1 for13C6IAA. Cone voltage was 18 V and collision energy was 18 V. Dwell time was 161 ms per channel.
Data were analysed using MassLynx software. IAA and13C6IAA eluted at 3.74 mins under these conditions.
Gene expression analysis
'Royal Gala' apple fruit were harvested in the year 2006-07 from the Plant & Food Research Orchard, Hawke's Bay. For flowers, whole flowers were sampled; all fertilised apples had seed removed before harvesting. As the apples developed, sections of tissue from at least 10 apples were harvested (containing skin, cortex and core) into liquid nitrogen. RNA extraction and cDNA was generated as described in [30].Gene expression was measured using quantitative PCR (qPCR) using Power SYBR®green probes (Applied Biosystems, UK). Primers were used that gave a single melting peak for each of the genes assessed (Primer sequences can be found in Additional file4).Quantitative PCR was conducted across two instruments:ARFandTIRgene expression were performed on a Roche LightCycler®480™ with the set up according to [63], whereasABP1, PIN, GH3andAux/IAAwere assessed using an ABI PRISM®7900 HT Sequence Detection System (Applied Biosystems) according to the method described in [64].In all cases,actinwas used as a reference gene.
Mapping fruit size
Two F1progenies were used to study the fruit size. The first contained a duplicated population of 590 seedlings from a 'Royal Gala' and 'Braeburn' cross (RGxBB), grafted onto 'M.9' rootstock, located at two locations in New Zealand (PRF research orchards in Havelock North and Motueka). The second contained a duplicated population of 123 seedlings from a 'Starkrimson' and 'Granny Smith' cross (STK×GS) grafted on the semi-dwarfing rootstock 'Pajam 1' located in France (Melgueil INRA Montpellier Experimental station). For the RGxBB seedlings, two seasons of apples were assessed. Apples were thinned to a low crop load of 4 fruit per cm2of trunk size and a minimum of five representative fruit sizes were weighed at harvest. For the STK×GS population, no thinning was undertaken. Five seasons of total apple crop on each tree were harvested weighed and counted, and the average weight of apple calculated per year.
Analysis of variance and linear models (in R software v.2.9.2 - R Development Core Team, 2009 [65]) were used to assess genetic and environmental regulation of fruit size at each site over the number of seasons measured. QTL analyses were performed using the RGxBB parental genetic maps and on the STKxGS consensus map [66,67].JoinMap 3.0 [68] was used for constructing linkage maps. Fruit weight QTL intervals for each population were defined based on the peaks LOD-1 and LOD-2.
For additional gene mapping, PCR primer pairs were designed using Primer 3 Plus software (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi), to select 100-200 bp fragments spanning a putative SNP (Single Nucleotide Polymorphism). High Resolution Melting (HRM) analysis was used for the detection of DNA polymorphisms [69], performed on a LightCycler 480®, as described in [70].
The list of predicted gene transcripts present within the QTL interval was extracted from GDR (http://www.rosaceae.org/projects/apple_genome),我们现在成对将的结果n of theMalusxdomesticagenome predicted genes against theArabidopsisTAIR10_pep_20100802 database using BLASTP with an EXP cut-off < 1e-30.
References
- 1.
Given NK, Venis MA, Gierson D: Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta. 1988, 174 (3): 402-406. 10.1007/BF00959527.
- 2.
Nitsch JP: Growth and morphogenesis of the strawberry as related to auxin. Am J Bot. 1950, 37: 211-215. 10.2307/2437903.
- 3.
Gorguet B, Van Heusden AW, Lindhout P: Parthenocarpic fruit development in tomato. Plant Biol. 2005, 7 (2): 131-139. 10.1055/s-2005-837494.
- 4.
Agusti M, Almela V, Andreu I, Juan M, Zacarias L: Synthetic auxin 3,5,6-TPA promotes fruit development and climacteric in Prunus persica L. Batsch. J Hortic Sci Biotechnol. 1999, 74 (5): 556-560.
- 5.
Stern RA, Flaishman M, Applebaum S, Ben-Arie R: Effect of synthetic auxins on fruit development of 'Bing' cherry (Prunus avium L.). Sci Hortic (Amsterdam). 2007, 114 (4): 275-280. 10.1016/j.scienta.2007.07.010.
- 6.
Perrot-Rechenmann C, Napier RM: Auxins. Vitam Horm. 2005, 72: 203-233.
- 7.
Woodward AW, Bartel B: Auxin: regulation, action, and interaction. Ann Bot. 2005, 95 (5): 707-735. 10.1093/aob/mci083.
- 8.
Zhao Y: Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010, 61: 49-64. 10.1146/annurev-arplant-042809-112308.
- 9.
Staswick PE, Tiryaki I, Rowe ML: Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002, 14 (6): 1405-1415. 10.1105/tpc.000885.
- 10.
Bottcher C, Keyzers RA, Boss PK, Davies C: Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot. 2010, 61 (13): 3615-3625. 10.1093/jxb/erq174.
- 11.
Ludwig-Müller J: Auxin conjugates: Their role for plant development and in the evolution of land plants. J Exp Bot. 2011, 62 (6): 1757-1773. 10.1093/jxb/erq412.
- 12.
Zazímalová E, Murphy AS, Yang H, Hoyerová K, Hosek P: Auxin transporters--why so many?. Cold Spring Harbor perspectives in biology. 2010, 2 (3): a001552-10.1101/cshperspect.a001552.
- 13.
Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K: Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998, 282 (5397): 2226-2230.
- 14.
Nishio S, Moriguchi R, Ikeda H, Takahashi H, Fujii N, Guilfoyle TJ, Kanahama K, Kanayama Y: Expression analysis of the auxin efflux carrier family in tomato fruit development. Planta. 2010, 232 (3): 755-764. 10.1007/s00425-010-1211-0.
- 15.
Abel S, Theologis A: Odyssey of auxin. Cold Spring Harb Perspect Biol. 2010, 2 (10): a004572-10.1101/cshperspect.a004572.
- 16.
Chapman EJ, Estelle M: Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet. 2009, 43: 265-285. 10.1146/annurev-genet-102108-134148.
- 17.
Shishova M, Lindberg S: A new perspective on auxin perception. J Plant Physiol. 2010, 167 (6): 417-422. 10.1016/j.jplph.2009.12.014.
- 18.
Tromas A, Paponov I, Perrot-Rechenmann C: Auxin binding protein 1: functional and evolutionary aspects. Trends Plant Sci. 2010, 15 (8): 436-446. 10.1016/j.tplants.2010.05.001.
- 19.
Parry G, Estelle M: Auxin receptors: A new role for F-box proteins. Curr Opin Cell Biol. 2006, 18 (2): 152-156. 10.1016/j.ceb.2006.02.001.
- 20.
Leblanc N, David K, Grosclaude J, Pradier JM, Barbier-Brygoo H, Labiau S, Perrot-Rechenmann C: A novel immunological approach establishes that the auxin-binding protein, Nt-abp1, is an element involved in auxin signaling at the plasma membrane. J Biol Chem. 1999, 274 (40): 28314-28320. 10.1074/jbc.274.40.28314.
- 21.
Tromas A, Braun N, Muller P, Khodus T, Paponov IA, Palme K, Ljung K, Lee JY, Benfey P, Murray JA, et al: The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth. PLoS One. 2009, 4 (9): e6648-10.1371/journal.pone.0006648.
- 22.
David KM, Couch D, Braun N, Brown S, Grosclaude J, Perrot-Rechenmann C: The auxin-binding protein 1 is essential for the control of cell cycle. Plant J. 2007, 50 (2): 197-206. 10.1111/j.1365-313X.2007.03038.x.
- 23.
Chen JG, Ullah H, Young JC, Sussman MR, Jones AM: ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev. 2001, 15 (7): 902-911. 10.1101/gad.866201.
- 24.
Balbi V, Lomax TL: Regulation of early tomato fruit development by the diageotropica gene. Plant Physiol. 2003, 131 (1): 186-197. 10.1104/pp.010132.
- 25.
Christian M, Steffens B, Schenck D, Lüthen H: The diageotropica mutation of tomato disrupts a signalling chain using extracellular auxin binding protein 1 as a receptor. Planta. 2003, 218 (2): 309-314. 10.1007/s00425-003-1090-8.
- 26.
de Jong M, Wolters-Arts M, Feron R, Mariani C, Vriezen WH: The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 2009, 57 (1): 160-170. 10.1111/j.1365-313X.2008.03671.x.
- 27.
Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latche A, Pech JC, Bouzayen M: The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell. 2005, 17 (10): 2676-2692. 10.1105/tpc.105.033415.
- 28.
Guillon F, Philippe S, Bouchet B, Devaux MF, Frasse P, Jones B, Bouzayen M, Lahaye M: Down-regulation of an Auxin Response Factor in the tomato induces modification of fine pectin structure and tissue architecture. J Exp Bot. 2008, 59 (2): 273-288. 10.1093/jxb/erm323.
- 29.
Jones B, Frasse P, Olmos E, Zegzouti H, Li ZG, Latche A, Pech JC, Bouzayen M: Down-regulation of DR12, an auxin-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J. 2002, 32 (4): 603-613. 10.1046/j.1365-313X.2002.01450.x.
- 30.
詹森BJ, Thodey K,谢弗RJ Alba R,Balakrishnan L, Bishop R, Bowen JH, Crowhurst RN, Gleave AP, Ledger S, et al: Global gene expression analysis of apple fruit development from the floral bud to ripe fruit. BMC Plant Biol. 2008, 8: 16-10.1186/1471-2229-8-16.
- 31.
Mousdale DMA, Knee M: Indolyl-3-acetic acid and ethylene levels in ripening apple fruits. J Exp Bot. 1981, 32 (4): 753-758. 10.1093/jxb/32.4.753.
- 32.
Treharne KJ,昆兰JD,奈特约,病房DA: Hormonal regulation of fruit development in apple: 'A mini-review'. Plant Growth Regul. 1985, 3 (2): 125-132. 10.1007/BF01806052.
- 33.
Newcomb RD, Crowhurst RN, Gleave AP, Rikkerink EH, Allan AC, Beuning LL, Bowen JH, Gera E, Jamieson KR, Janssen BJ, et al: Analyses of expressed sequence tags from apple. Plant Physiol. 2006, 141 (1): 147-166. 10.1104/pp.105.076208.
- 34.
Schaffer RJ, Friel EN, Souleyre EJ, Bolitho K, Thodey K, Ledger S, Bowen JH, Ma JH, Nain B, Cohen D, et al: A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol. 2007, 144 (4): 1899-1912. 10.1104/pp.106.093765.
- 35.
Johnston JW, Gunaseelan K, Pidakala P, Wang M, Schaffer RJ: Co-ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. J Exp Bot. 2009, 60 (9): 2689-2699. 10.1093/jxb/erp122.
- 36.
Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC: Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007, 49 (3): 414-427. 10.1111/j.1365-313X.2006.02964.x.
- 37.
Atkinson RG, Schroder R, Hallett IC, Cohen D, MacRae EA: Overexpression of polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol. 2002, 129 (1): 122-133. 10.1104/pp.010986.
- 38.
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, et al: The genome of the domesticated apple (Malus × domestica Borkh.). Nat Genet. 2010, 42 (10): 833-839. 10.1038/ng.654.
- 39.
Gustafson FG: Auxin distribution in fruits and its significance in fruit development. Am J Bot. 1939, 26: 189-194. 10.2307/2436487.
- 40.
Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, Jaiswal P, Mockaitis K, Liston A, Mane SP, et al: The genome of woodland strawberry (Fragaria vesca). Nat Genet. 2011, 43 (2): 109-116. 10.1038/ng.740.
- 41.
Remington DL, Vision TJ, Guilfoyle TJ, Reed JW: Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Physiol. 2004, 135 (3): 1738-1752. 10.1104/pp.104.039669.
- 42.
Kalluri UC, Difazio SP, Brunner AM, Tuskan GA: Genome-wide analysis of Aux/IAA and ARF gene families in Populus trichocarpa. BMC Plant Biol. 2007, 7: 59-10.1186/1471-2229-7-59.
- 43.
Wang Y, Deng D, Bian Y, Lv Y, Xie Q: Genome-wide analysis of primary auxin-responsive Aux/IAA gene family in maize (Zea mays. L.). Mol Biol Rep. 2010, 37 (8): 3991-4001. 10.1007/s11033-010-0058-6.
- 44.
Xing H, Pudake RN, Guo G, Xing G, Hu Z, Zhang Y, Sun Q, Ni Z: Genome-wide identification and expression profiling of auxin response factor (ARF) gene family in maize. BMC Genomics. 2011, 12: 178-10.1186/1471-2164-12-178.
- 45.
Kumar R, Tyagi AK, Sharma AK: Genome-wide analysis of auxin response factor (ARF) gene family from tomato and analysis of their role in flower and fruit development. Mol Genet Genomics. 2011, 285 (3): 245-260. 10.1007/s00438-011-0602-7.
- 46.
Srivastava A, Handa AK: Hormonal regulation of tomato fruit development: A molecular perspective. J Plant Growth Regul. 2005, 24 (2): 67-82. 10.1007/s00344-005-0015-0.
- 47.
Liebhard R, Kellerhals M, Pfammatter W, Jertmini M, Gessler C: Mapping quantitative physiological traits in apple (Malus × domestica Borkh.). Plant Mol Biol. 2003, 52 (3): 511-526. 10.1023/A:1024886500979.
- 48.
Bertin N, Causse M, Brunel B, Tricon D, Génard M: Identification of growth processes involved in QTLs for tomato fruit size and composition. J Exp Bot. 2009, 60 (1): 237-248.
- 49.
Grandillo S, Ku HM, Tanksley SD: Identifying the loci responsible for natural variation in fruit size and shape in tomato. Theor Appl Genet. 1999, 99 (6): 978-987. 10.1007/s001220051405.
- 50.
Harada T, Kurahashi W, Yanai M, Wakasa Y, Satoh T: Involvement of cell proliferation and cell enlargement in increasing the fruit size of Malus species. Sci Hortic. 2005, 105 (4): 447-456. 10.1016/j.scienta.2005.02.006.
- 51.
McAtee PA, Hallett IC, Johnston JW, Schaffer RJ: A rapid method of fruit cell isolation for cell size and shape measurements. Plant Methods. 2009, 5 (1): 5-10.1186/1746-4811-5-5.
- 52.
Malladi A, Hirst PM: Increase in fruit size of a spontaneous mutant of 'Gala' apple (Malus × domestica Borkh.) is facilitated by altered cell production and enhanced cell size. J Exp Bot. 2010, 61 (11): 3003-3013. 10.1093/jxb/erq134.
- 53.
Mallory AC, Bartel DP, Bartel B: MicroRNA-directed regulation of Arabidopsis Auxin Response Factor17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell. 2005, 17 (5): 1360-1375. 10.1105/tpc.105.031716.
- 54.
Trewavas AJ, Cleland RE: Is plant development regulated by changes in the concentration of growth substances or by changes in the sensitivity to growth substances?. Trends Biochem Sci. 1983, 8 (10): 354-357. 10.1016/0968-0004(83)90359-6.
- 55.
Stern RA, Ben-Arie R: Pre-harvest drop control of 'Red Delicious' and 'Jonathan' apple (Malus domestica) as affected by the synthetic auxin 3,5,6-TPA. J Hortic Sci Biotech. 2006, 81 (6): 943-948.
- 56.
Percy AE, Jameson PE, Melton LD: Expansion during early apple fruit development induced by auxin and N-(2-chloro-4-pyridyl)-N'-phenylurea: effect on cell wall hemicellulose. Plant Growth Regul. 1998, 26 (1): 1-6. 10.1023/A:1006032302995.
- 57.
Goetz M, Vivian-Smith A, Johnson SD, Koltunow AM: AUXIN RESPONSE FACTOR8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell. 2006, 18 (8): 1873-1886. 10.1105/tpc.105.037192.
- 58.
Goetz M, Hooper LC, Johnson SD, Rodrigues JC, Vivian-Smith A, Koltunow AM: Expression of aberrant forms of AUXIN RESPONSE FACTOR8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol. 2007, 145 (2): 351-366. 10.1104/pp.107.104174.
- 59.
Zhang J, Chen R, Xiao J, Qian C, Wang T, Li H, Ouyang B, Ye Z: A single-base deletion mutation in SlIAA9 gene causes tomato (Solanum lycopersicum) entire mutant. J Plant Res. 2007, 120 (6): 671-678. 10.1007/s10265-007-0109-9.
- 60.
刘K,公元前康,江H,摩尔SL,李H,沃特金斯CB, Setter TL, Jahn MM: A GH3-like gene, CcGH3, isolated from Capsicum chinense L. fruit is regulated by auxin and ethylene. Plant Mol Biol. 2005, 58 (4): 447-464. 10.1007/s11103-005-6505-4.
- 61.
Trainotti L, Tadiello A, Casadoro G: The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J Exp Bot. 2007, 58 (12): 3299-3308. 10.1093/jxb/erm178.
- 62.
Chaabouni S, Jones B, Delalande C, Wang H, Li Z, Mila I, Frasse P, Latche A, Pech JC, Bouzayen M: Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signalling involved in differential growth. J Exp Bot. 2009, 60 (4): 1349-1362. 10.1093/jxb/erp009.
- 63.
Tacken E, Ireland H, Gunaseelan K, Karunairetnam S, Wang D, Schultz K, Bowen J, Atkinson RG, Johnston JW, Putterill J, et al: The role of ethylene and cold temperature in the regulation of the apple POLYGALACTURONASE1 gene and fruit softening. Plant Physiol. 2010, 153 (1): 294-305. 10.1104/pp.109.151092.
- 64.
Günl M, Liew EF, David K, Putterill J: Analysis of a post-translational steroid induction system for GIGANTEA in Arabidopsis. BMC Plant Biology. 2009, 9: 141-10.1186/1471-2229-9-141.
- 65.
R development Core team: R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna: R Foundation for Statistical Computing. 2009.
- 66.
Segura V, Denancé C, Durel CE, Costes E: Wide range QTL analysis for complex architectural traits in a 1-year-old apple progeny. Genome. 2007, 50 (2): 159-171. 10.1139/G07-002.
- 67.
Guitton B, Kelner JJ, Velasco R, Gardiner SE, Chagné D, Costes E: Genetic control of biennial bearing in apple. J Exp Bot. 2012, 63 (1): 131-149. 10.1093/jxb/err261.
- 68.
van Oojen J, Voorips R: JoinMap 3.0, Software for the calculation of genetic linkage maps. Plant Research International. Wageningen: The Netherlands; 2001.
- 69.
Liew M, Pryor R, Palais R, Meadows C, Erali M, Lyon E, Wittwer C: Genotyping of single-nucleotide polymorphisms by high-resolution melting of small amplicons. Clin Chem. 2004, 50 (7): 1156-1164. 10.1373/clinchem.2004.032136.
- 70.
Chagne D, Gasic K, Crowhurst RN, Han Y, Bassett HC, Bowatte DR, Lawrence TJ, Rikkerink EH, Gardiner SE, Korban SS: Development of a set of SNP markers present in expressed genes of the apple. Genomics. 2008, 92 (5): 353-358. 10.1016/j.ygeno.2008.07.008.
Acknowledgements
This work was supported by a Faculty Research Development Fund from the University of Auckland and a New Zealand Foundation for Research Science and Technology grant, contract number C06X0705. We thank Noel Davies for UPLC measurements and the Australian Research Council's Linkage Infrastructure and Equipment Funding scheme (project number LE10010015).
Author information
Affiliations
Corresponding author
Additional information
Authors' contributions
Experimental procedures were performed by FD, TD, WP, JK, KG (extracted RNA, screened the genome for auxin-related genes and performed expression analysis). JRR, TJL measured auxin content, ICH undertook the microscopy assessment, KCB, GAD harvested apples and assessed the fruit, GAD, RD, DST set up the RGxBB cross and conducted fruit weight assessments on this population BG, EC, and DC constructed the genetic map and identified QTLs for fruit weight. RJS and KMD conceived the project and analysed the data; DC, RJS and KMD wrote the paper. All authors read and approved the final manuscript.
电子年代upplementary material
List of auxin-related genes in apples
Additional file 1: . Table of Predicted Apple genes by MDP number [38], designated names and chromosome location (Gene and protein sequences can be obtained from GDR:http://www.rosaceae.org).(DOC 189 KB)
12870_2011_975_MOESM2_ESM.DOCX
Additional file 2: Accession numbers of proteins sequences from other species used to build phylogenetic tree. (DOCX 19 KB)
Phylogenetic trees for PIN, GH3 and Aux/IAA class of genes
Additional file 3: . Protein sequences of PIN, GH3, Aux/IAA from apple (green), strawberry (lilac),Arabidopsis(black) and tomato (red) were aligned using MUSCLE and phylogenetic trees were built using neighbour joining. Bootstraps of 1000 iterations are given.At: Arabidopsis thaliana, Fv: Fragaria vesca, Md: Malusxdomestica, Pp: Physcomitrella patens, Sl: Solanum lycopersicu. (DOCX 343 KB)
12870_2011_975_MOESM4_ESM.XLSX
Additional file 4: List of qPCR primers used to measure gene expression patterns, and relative expression used for clustering. (XLSX 21 KB)
Genes identified within QTL interval for fruit weight on Ch15
Additional file 6: . List of the 133 predicted gene transcripts present within the QTL interval for fruit weight on LG15. The table displays gene location and putative function based on homology to known genes inArabidopsis thaliana(pairwise comparison of theMalusxdomesticagenome predicted genes against theArabidopsisTAIR10_pep_20100802 database using BLASTP with an EXP cut-off < 1e-30). Data were retrieved from the Genome Database for Rosaceae (http://www.rosaceae.org/projects/apple_genome).(XLSX 37 KB)
作者的起源nal submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open AccessThis article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Devoghalaere, F., Doucen, T., Guitton, B.et al。基因组学的方法来理解的角色uxin in apple (Malusxdomestica)fruit size control.BMC Plant Biol12,7 (2012). https://doi.org/10.1186/1471-2229-12-7
Received:
Accepted:
Published:
Keywords
- Fruit Development
- Fruit Weight
- Fruit Size
- Apple Fruit
- Auxin Concentration