- Research article
- Open Access
- Published:
Molecular characterization ofTaSTOP1homoeologues and their response to aluminium and proton (H+) toxicity in bread wheat (Triticum aestivumL.)
BMC Plant Biologyvolume13, Article number:134(2013)
Abstract
Background
Aluminium (Al) toxicity is considered to be one of the major constraints affecting crop productivity on acid soils. Being a trait governed by multiple genes, the identification and characterization of novel transcription factors (TFs) regulating the expression of entire response networks is a very promising approach. Therefore, the aim of the present study was to clone, localize, and characterize theTaSTOP1gene, which belongs to the zinc finger family (Cys2His2 type) transcription factor, at molecular level in bread wheat.
Results
TaSTOP1loci were cloned and localized on the long arm of homoeologous group 3 chromosomes [3AL (TaSTOP1-A), 3BL (TaSTOP1-B) and 3DL (TaSTOP1-D)] in bread wheat. TaSTOP1 showed four potential zinc finger domains and the homoeologue TaSTOP1-A exhibited transactivation activity in yeast. Expression profiling ofTaSTOP1transcripts identified the predominance of homoeologueTaSTOP1-Afollowed byTaSTOP1-DoverTaSTOP1-Bin root and only predominance ofTaSTOP1-Ain shoot tissues of two diverse bread wheat genotypes. Al and proton (H+) stress appeared to slightly modulate the transcript ofTaSTOP1homoeologues expression in both genotypes of bread wheat.
Conclusions
Physical localization ofTaSTOP1results indicated the presence of a single copy ofTaSTOP1on homoeologous group 3 chromosomes in bread wheat. The three homoeologues ofTaSTOP1have similar genomic structures, but showed biased transcript expression and different response to Al and proton (H+) toxicity. These results indicate thatTaSTOP1homoeologues may differentially contribute under Al or proton (H+) toxicity in bread wheat. Moreover, it seems thatTaSTOP1-Atransactivation potential is constitutive and may not depend on the presence/absence of Al at least in yeast. Finally, the localization ofTaSTOP1on long arm of homoeologous group 3 chromosomes and the previously reported major loci associated with Al resistance at chromosome 3BL, through QTL and genome wide association mapping studies suggests thatTaSTOP1could be a potential candidate gene for genomic assisted breeding for Al tolerance in bread wheat.
Background
Aluminium (Al) toxicity is one of the major concerns for crop productivity in acidic soil, accounting for more than 50% of the global arable land [1]。Al is one of the highly abundant elements in the earth crust and under low pH conditions (acidic soils), it is solubilised in the soil in a toxic ionic form that inhibits root elongation. The primary response of plants to Al toxicity is the rapid inhibition of root growth, particularly in the root apex by blocking the process of cell division along with cell elongation, and subsequently inefficient absorption of nutrients and water from soil, resulting in the reduction of plant growth and overall productivity [2]。
Plant species differ in the level of Al resistance because they evolved different mechanisms to overcome the selective pressure of Al toxicity imposed under acid soils [3]。These mechanisms can be broadly divided into two categories: Al resistance and Al tolerance. The Al resistance mechanism is often based on the Al exclusion from the root apex or the external detoxification of Al in the rhizosphere through the release of organic acid anions (oxalate, citrate and malate) from the roots limiting Al uptake [4]。On the other hand, the tolerance mechanism may involve the entrance of Al through the roots and its relocation (in the vacuole) or internal detoxification through Al chelating with organic acids (citrate and oxalate) [5]。These mechanisms have been validated at the molecular level, particularly with the functional characterization of the major genes such asALMT(Aluminium-Activated Malate Transporter) andMATE(Multidrug and Toxic compound Exudation) in bread wheat (Triticum aestivumL.) [6] and sorghum (Sorghum bicolorL. Moench) [7], respectively. In addition, genes encoding transporters have been identified through mutational analysis, especially ABC transporters type (ALS1andALS3) inArabidopsis[8,9] and bacterial-type ABC transporters (STAR1andSTAR2) in rice (Oryza sativaL.) [10]。Recently, a plasma membrane localized transporterNrat1(Nramp aluminum transporter 1) was also found to be associated with Al tolerance particularly to trivalent form of Al in rice [11]。These genes are essential for Al resistance, and seem to be a part of the pathway involved in the secondary level of protection through uptake and redistribution of Al to less sensitive tissues in plants, although their actual function in Al tolerance mechanism in plant is still unclear.
There is strong evidence supporting the important role of regulatory genes (transcriptional factors) in plant tolerance to abiotic stresses [12–15]。Recently, the zinc finger transcription factors,STOP1(sensitive to proton rhizotoxicity) andART1(Al resistance transcription factor) have been identified in Al sensitive mutants ofArabidopsisand rice, respectively [16,17]。Transcriptome analysis under Al stress revealed that 101 and 31 genes were down-regulated in the Arabidopsisstop1[18] and riceart1mutants [17], respectively. Interestingly, the major genes related to Al tolerance, particularlyALMT1andMATE1were regulated by these transcription factors [18,19]。Recently, the contribution of ART1 locus to the variation for Al tolerance in rice has also been identified in QTL analysis [20]。The limited impact of single functional genes in plant stress tolerance has been associated with the polygenic nature of such traits. Thus, the identification and characterization of key regulatory genes that act as master regulators controlling entire response networks would be the most promising and sustainable approach to modify complex traits in plants as they coordinate the expression of many target genes [21]。
小麦是最重要的一个自然allopolyploid species, as it is not only directly or indirectly contributing in the food supply for nearly half of the worlds’ population but also can serve as a model plant for other economically important polyploid crop species. It is considered as one of the sensitive crop to Al stress among cereals. Bread wheat, with hexaploid nature comprised from three genomes (AABBDD) are organized in seven homoeologous groups, each homoeologous group has individual gene in triplicate form (from each of A, B and D genomes). Consequently, it is of great interest to reveal how the expressions of homoeologues genes are regulated in hexaploid wheat because theoretically, all the three homoeologues of a gene are assumed to be uniformly expressed. In the previous decade, several studies have been conducted in order to identify the molecular markers (random DNA markers) linked to Al tolerance through QTL mapping and genome-wide association analyses [22–26]。到目前为止,只有两个候选基因ALMT1和MATE1for Al tolerance in wheat have been identified and also mapped on chromosome 4DL using Chinese Spring deletion lines and 4BL through QTL mapping, respectively [22,27,28]。Recently, the three homoeologues of TaMATE1 have been cloned [29], although, no information is available on the expression of respective homoeologues of these candidate genes for Al tolerance in hexaploid wheat. Therefore, in order to improve Al tolerance in bread wheat, identification of three homoeologues of the candidate gene is a promising strategy that could be utilized to develop functional markers for genomic assisted breeding programme in wheat. Herein, we report on the identification, physical localization, and molecular characterization of a novel transcription factorTaSTOP1homoeologues genes in bread wheat for Al and proton (H+) tolerance.
Results
Cloning and structure ofTaSTOP1
In order to clone theSTOP1in wheat, primers from wheat EST showing highest similarity withArabidopsis thaliana STOP1were used to amplify theTaSTOP1in bread wheat genotype Barbela 7/72/92. Further, 5’ and 3’ UTR ends ofTaSTOP1were amplified using RACE as described in material and methods.TaSTOP1was amplified in six different bread wheat genotypes and its multiple alignments suggested the mixed amplification from distinct wheat genomes (A, B, and D). Moreover, the comparison of theTaSTOP1cDNA sequence with genomic sequence revealed thatTaSTOP1gene does not contain introns.
The coding region ofTaSTOP1(TaSTOP1-A) from genome A has 1533 bp length and is differentiated from genomes B (TaSTOP1-B) and D (TaSTOP1-D) by having a 6-bp deletion (1276–1281 positions).TaSTOP1-BandTaSTOP1-D are differentiated by several SNPs in the respective open reading frame (ORF) [Additional file1]。TaSTOP1-Din bread wheat genotype Barbela 7/72/92 has a full-length cDNA of 1970 bp, containing a coding region of 1539 bp that encodes a polypeptide of 512 amino acids (Figure1).TaSTOP1-Dhas a molecular weight of 55.8 kD, with a 5.64 isoelectric point (pI). InterProScan function domain analysis suggests that TaSTOP1 belongs to the Cys2His2 zinc finger family protein. Subcellular prediction analysis indicated that TaSTOP1 protein is localized in the nucleus.
Phylogenetic studies for STOP1 gene among plant species
Deduced amino acid sequence of TaSTOP1 consists of 510 (TaSTOP1-A) and 512 (TaSTOP1-B and TaSTOP1-D) amino acids in bread wheat and the phylogenetic relationship with other STOP-like proteins from 32 different plant species was studied based on their full length sequences. Phylogenetic analysis of STOP like proteins clearly formed two distinct clusters which implied that STOP2 (AT5G22890) was completely distinct from STOP1 (AT1G34370) in Arabidopsis. Furthermore, STOP1 and STOP2 protein from monocotyledons can be clearly distinguished from eudicots (Figure2A). TaSTOP1 homoeologues from bread wheat showed high similarity with STOP1 from Barley (Hordeum vulgare) andBrachypodium distachiumwith identities of 92 and 87%, respectively. Noticeably, the phylogenetic relationship of TaSTOP1 homoeologoues particularly TaSTOP1-A and TaSTOP1-D from bread wheat (AABBDD) displayed the maximum similarity with STOP1 like proteins fromTriticum urartu(AA) andAegilops tauschii(DD), respectively (Figure2A).
系统发育树和多个poten比对tial Zinc Fingers domain of STOP-like proteins.Phylogenetic tree based on amino acid sequences showing the relationship of TaSTOP1 with other plant STOP1 type proteins(A)。The deduced amino acid sequences were aligned with CLUSTALW. Comparison of ZFs domain of TaSTOP1 (TaSTOP1-A, TaSTOP1-B, TaSTOP1-D) with its homologous from Brachypodium (B.distachyon), Barley (H.vulgare), rice (O.sativa), sorghum (S.bicolor), maize (Z.mays) foxmillet (S.italica), and Arabidopsis (A.thaliana1 &A.thaliana2)(B)。Horizontal bars indicate ZFs domain and asterisks indicate conserved motif of Cys2His2 or Cys2His2-Cys.
Sequence alignment of STOP1 protein from bread wheat with its homologous from cereals [Brachipodium,barley, rice, sorghum, maize (Zea mays) and foxmillet (Setaria italic)] andArabidopsis, shows thatTaSTOP1encodes a putative Cys2His2 zinc finger protein containing four potential zinc finger domains. Three zinc finger domains (ZF1, ZF2, and ZF4) are predicted as the Cys2His2 type, whereas ZF3 is predicted as the Cys2His-Cys or the Cys2His2 type (Figure2B). Furthermore, sequence alignment revealed that STOP-like proteins share highly conserved regions in the ZF domains. In addition, the STOP1 protein also showed greater sequence conservation in the C-terminus than in the N-terminus among family members. The 6-bp deletion in TaSTOP1-A resulted in a loss of two amino acids [Pro (P) and Gln (Q)] at position 426 without changing the reading frame [Additional file2]。We also observed in Barbela 7/72/92, two SNPs at position 221 (A replaced by G) and 1186 (G replaced by A) which substituted an Asn (N) by a Ser (S) and Asp (D) by Asn (N) inTaSTOP1-Bthan in bothTaSTOP1-AandTaSTOP1-Dhomoeologues, respectively [Additional file1]。Multiple alignment of STOP1 protein revealed that the SNP at position 1186 is located in the zinc finger domain (ZF4) of TaSTOP1 homoeologues (Figure2B).
TaSTOP1localization on distinct wheat genomes
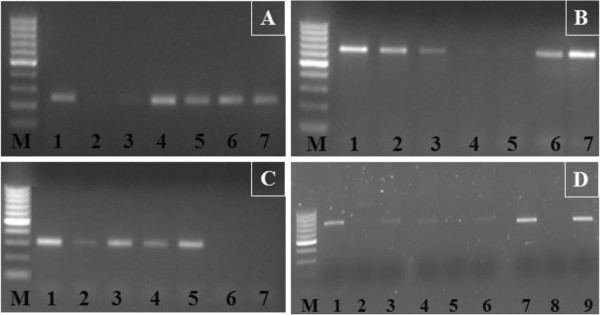
For localization ofTaSTOP1on homoeologous chromosomes, locus specific primer pairs were designed [Additional file3] andTaSTOP1homoeologues were amplified in a series of nullitetrasomic lines of Chinese Spring wheat along with Chinese Spring as a positive control. On the basis of the presence or absence of PCR products visualized in agarose gels, we observed thatTaSTOP1genes are located on homoeologous chromosomes 3A, 3B and 3D, and namedTaSTOP1-A, TaSTOP1-BandTaSTOP1-D, respectively (Figure3A-C). Furthermore, ditelosomic lines of Chinese Spring for homoeologous group 3 chromosomes were also used to assign theTaSTOP1on chromosomal arms and confirmed thatTaSTOP1-A,TaSTOP1-BandTaSTOP1-D部分同源的基因位于长臂chromosomes 3A, 3B and 3D, respectively (Figure3D).
TaSTOP1mapping on homoeologous chromosomes and their arms in bread wheat.TaSTOP1mapping on wheat chromosomes from genome A(A), B(B)and D(C)using nullitetrasomic lines. M: Molecular-weight marker (100bp ladder), 1 to 7: Chinese Spring as control, N3AT3B, N3AT3D, N3BT3A, N3BT3D, N3DT3A, N3DT3B. Arms mapping ofTaSTOP1-A,TaSTOP1-BandTaSTOP1-Dwith ditelosomic lines of homoeologous group 3(D)。1、4和7:中国弹簧控制;2: Dt3AS; 3: Dt3AL; 5: Dt3BS; 6: Dt3BL; 8: Dt3DS and 9: Dt3DL.
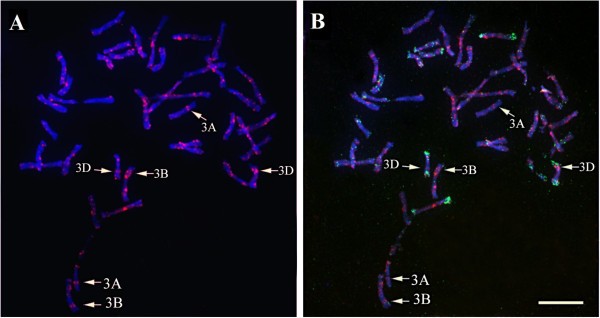
In order to further validate these results, physical chromosomal localization ofTaSTOP1was performed using Tyramide Signal Amplification FISH (Tyr-FISH) technique in the root-tip metaphase chromosome spreads of bread wheat genotype Barbela 7/72/92. For this purpose, a probe was developed by PCR amplification ofTaSTOP1from genomic DNA of Barbela 7/72/92. FISH with theTaSTOP1specific probe on metaphase chromosomes depicted hybridization signals ofTaSTOP1on both chromatids of the long arms of three chromosomes (Figure4A) and simultaneous re-probing of chromosome preparations with GAA- (red) and pAs1 (green) identified homoeologous group 3 with positive signals on chromosomes 3AL, 3BL and 3DL (Figure4B). Some background was also observed but it was distinguished from true hybridization signals because they usually appear as dots without any pattern and not in both chromatids of the same chromosome. Only those signals having the same size and appearance on the same position of both chromatids of one specific chromosome were analyzed as true-positive hybridization signals.
Physical localization ofTaSTOP1on homoeologous chromosome in the root-tip spreads of bread wheat genotype Barbela 7/72/92 using TYR-FISH technique.(A)Detection of positive hybridization signals ofTaSTOP1on both chromatids of the long arms of three chromosomes(B)and identification of chromosomes after re-probing with GAA- (red) and pAs1 (green). Note: The GAA-satellite sequence identifies A and B genome chromosomes whereas the pAs1 identifies chromosomes from the D genome. Arrow indicates the position of TaSTOP1 on respective chromosome (Scale bar = 10 microm).
TaSTOP1 transactivation activity
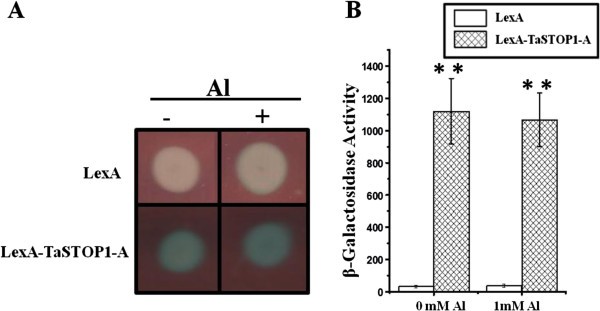
The nuclear localization of TaSTOP1 protein was predicted by WOLF-PSORT programme [30]。TaSTOP1is a putative transcription factor of the Cys2His2-type Zinc fingers family. The three homoeologues ofTaSTOP1showed similar genomic structure in bread wheat [Additional file2]。Therefore, we only proceed to evaluate the transactivation potential ofTaSTOP1located on genome A (TaSTOP1-A).To this aim, we performed a modified yeast one-hybrid assay [31] to evaluate the ability of the lexA-TaSTOP1-A fusion protein to activate the lexA-driven expression of thelacZgene in a heterologous yeast system, the results of which are illustrated in Figure5。It is shown that lexA-TaSTOP1-A has the potential to transactivatelacZ表达式的存在与否, further corroborating its potential role as a transcription factor.
LexA-TaSTOP1-A exhibits transactivation potential in yeast.EGY48 cells co-expressing the lexA-TaSTOP1-A and a report cassette bearing thelacZgene driven by a promoter containing lexA binding sites were grown in selective medium in the presence or absence of Al. Qualitative overlay-assay performed on solid medium(A)and quantitative measurements of β-galactosidase activity normalized to the A600of cells(B)。Values are means (± SD) of six independent transformants. Asterisks denote significant differences as identified by pairedt-tests (**: P < 0.001).
Expression profile of threeTaSTOP1homoeologues in bread wheat
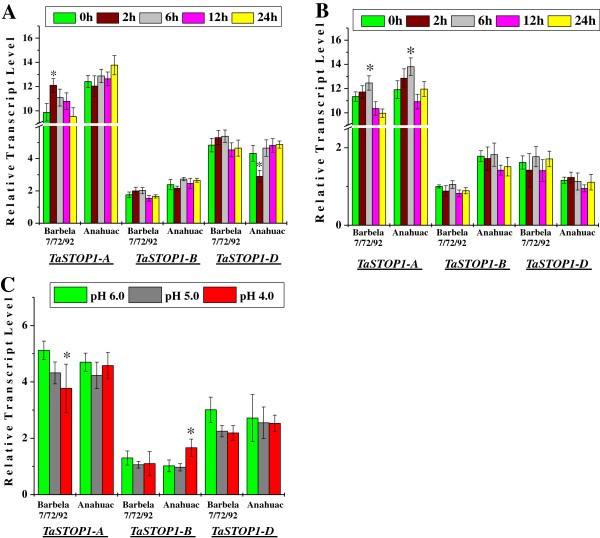
To understand the homoeologue specific expression ofTaSTOP1in bread wheat, we measured the temporal expression ofTaSTOP1-A, TaSTOP1-BandTaSTOP1-Dby Real-time qPCR in the root and shoot tissues of two bread wheat genotypes [Barbela 7/72/92 (Al resistant) and Anahuac (Al sensitive)] grown under Al stress (74 μM Al). The 18SRNA前女友pression was used as an internal control. We noticed a biased transcription of homoeologues ofTaSTOP1gene in the root and shoot tissue of these diverse bread wheat genotypes (Figure6).The transcript levels of homoeologueTaSTOP1-Aunder control as well as Al treatment were significantly higher than those ofTaSTOP1-BandTaSTOP1-Din root and shoot tissues of both genotypes (depending upon genotypes, fold differences in the transcript abundance were about 5–6 and 6–11 for homoeologueTaSTOP1-AversusTaSTOP1-Bin root and shoot, respectively) whereas expression of homoeologueTaSTOP1-Dwas only two-fold higher thanTaSTOP1-Bin the root tissues of both genotypes (Figure6A and B). A slight induction (within the two hours of Al exposure) followed by return to basal levels of the homoeologueTaSTOP1-Atranscripts was observed in the root tissues of Barbela 7/72/92 (Al resistant) whereas in the Anahuac (Al sensitive) under Al stress it was observed a quite stable transcript expression (Figure6A). The expression ofTaSTOP1-Bwas also almost unaltered in the roots of both genotypes under Al stress. Similarly,TaSTOP1-Dwas also constitutively expressed under Al stress in the roots of both genotypes except a rapid (2 h) and significant repression in the Al sensitive genotype Anahuac (Figure6A). Nevertheless, a quite stable transcript expression ofTaSTOP1homoeologues was observed in the shoot of both genotypes except homoeologueTaSTOP1-A(Figure6B). Interestingly, and similarly to roots, the expression ofTaSTOP1-Ain the shoots of both genotypes was also slightly induced under Al stress, however, its up-regulation was noticed only after 6 h and return to its basal level (Figure6B). It is worth noting that the transcript levels ofTaSTOP1-Bof Al tolerant genotype Barbela 7/72/92 are lower in the shoot tissues, compared to the sensitive genotype Anahuac (Figure6B).
Relative transcript level (fold change) ofTaSTOP1homoeologues genes under Al stress in the roots (A) as well as shoots (B) and under proton (H+) stress in roots (C) of two bread wheat genotypes Barbela 7/72/92 and Anahuac.Among three homoeologues, the lowest Δ Ct value of aTaSTOP1homoeologue [TaSTOP1-Bin shoots of Barbela 7/72/92 (0 h; as control) and in roots of Anahuac (pH 6.0; as control) for Al and pH stress assay, respectively] was used as calibrator. Values are means (± SD) of three independent replicates. Asterisks indicate significant difference between the control and treatment sample in the respective genome of a genotype (Student´sttest, * P < 0.01).
Moreover, to evaluate the transcript expression ofTaSTOP1homoeologues under protons (H+) stress, we also performed the relative quantification of homoeologue specific expression ofTaSTOP1transcript in root tissues of both genotypes under different levels of pH (6.0, 5.0 and 4.0). A slight gradual decrease in the transcript levels ofTaSTOP1-Ahomoeologue was observed in the roots of Barbela 7/72/92 under protons (H+) stress, but a quite stable expression was observed in genotype Anahuac (Figure6C). Furthermore, considerable up-regulation of the homoeologueTaSTOP1-Band slight repression of the homoeologueTaSTOP1-Dtranscript level was also observed under low pH (pH 4.0) in the roots of genotype Anahuac and Barbela 7/72/92, respectively (Figure6C).
Discussion
So far onlyALMT1andMATEgenes have been described as responsible for most of the genotypic variation for Al tolerance and are considered as major genes for Al resistance in bread wheat [32]。These genes have been identified in wheat [6,27在模式植物拟南芥中,也19,33] and major cereals such as barley [34], maize [35], sorghum [7] and rye [36,37]。The mechanisms underlying Al-tolerance in plant species have yet to be fully elucidated, as it seems that Al targets multiple cellular sites such as cell walls, plasma membranes, and cellular processes, like signal transduction pathways and homeostasis mechanisms [2,5,38,39]。Recent evidence suggests that regulatory genes (transcription factors) play a key role in Al detoxification at different cellular levels [16,17]。
In the present study, a novel transcription factor gene named TaSTOP1 was cloned from an Al tolerant genotype Barbela 7/72/92 (Figure1) which was derived from Portuguese bread wheat landrace Barbela through single seed descent method [40]。TaSTOP1 belongs to a member of Cys2His2 zinc finger family proteins that contains four potential zinc finger domains and has highly conserved regions in the zinc finger (ZF) domains (Figure2B). Like other C2H2-type zinc finger proteins it contains more than one zinc finger motif with highly conserved amino acid sequence for DNA binding [16,41]。Phylogenetic analysis clearly differentiated the STOP1 like proteins from monocots in a group (Figure2A) and also suggested that genome A and B are more distant from genome D [Additional file2], as genome D was incorporated recently in comparison to the A and B genomes in bread wheat [42]。In addition, our phylogenetic analysis results also support previous findings at molecular level that the A and D genomes in bread wheat were derived fromT. urartuandA. tauschii, respectively [Additional file2] [42]。
In past, the nullitetrasomic and ditelosomic lines of Chinese Spring wheat have been extensively used in classical and molecular genetic studies for the identification of loci associated with numerous traits including Al tolerance [25,43]。Thus, gene mapping has practical implications in plant genetics and breeding, and the determination of its physical localization is even more precise as it enables us to confirm the exact position of a gene on a chromosome. PCR based mapping ofTaSTOP1using nullitetrasomic and ditellosomic lines of Chinese Spring wheat revealed thatTaSTOP1is located on the long arm of wheat homoeologous group 3 chromosomes (Figure3).
In situhybridization is not only the most direct method for physical localization of genes in chromosomes, but also it is a valuable tool for the identification of copy numbers of a gene in species having a highly complex genome such as the bread wheat. Due to its hexaploid nature, most genes can be found in triplicate with one copy on each genome [44–46]。Tyramide signal amplification FISH (Tyr-FISH) technique has been successfully used for the identification of low copy number DNA sequences in wheat [47]。However, for the successful localization of a gene using the FISH technique, the minimum length of the probe size seems to be crucial. Recently,RD50gene has been localized in bread wheat with a probe size of 2 kb [47]。Physical localization ofTaSTOP1by Tyr-FISH technique not only confirmed the results obtained from PCR based mapping, but also indicated thatTaSTOP1could be a single copy gene localized on the long arm of homoeologous group 3 chromosomes (Figure4).In bread wheat, physical localization of single copy geneGlu-1andRD50with FISH technique has been demonstrated on long and short arms of the homoeologous group 1 chromosomes, respectively [47,48]。In the present work, we were able for the first time to localize a gene with a probe size smaller than 2.0 kb demonstrating that the FISH technique can be used to simultaneously anchor homoeologous chromosomes with 1.5 kb probes even in bread wheat.
Subcellular prediction of TaSTOP1 protein in the nucleus is in agreement with data previously reported [18]。Zinc finger motifs are thought to recognize and bind to target DNA sequences, but they are not required for transcriptional activity [49,50]。Our results clearly exhibited the transactivation potential ofTaSTOP1-Aat least in yeast (Figure5).In present investigation, we observed highly similar genomic structure ofTaSTOP1genes [Additional files1and2], therefore, it seems thatTaSTOP1transactivation function is constitutive and may not depend on the presence/absence of Al. The SNPs observed amongTaSTOP1homoeologues showed minor changes in respective amino acids of putative proteins which could alter the secondary structures by influencing the folding of these proteins (Additional file1).Therefore, the three homoeologues of the same locus in bread wheat share high sequence similarity that could illustrate the flexibility of a polyploidy species in which due to the mutation in one homoeologue may be compensated for by the homoeologue [29]。
Allopolyploidy plays an important role in plant evolution that arises with the merging of two or more genomes into single nucleus which may contribute either equally or disproportionately. However, recent molecular findings confirmed the asymmetric genomic expression pattern in natural and synthetic allopolyploid plant species, but the predominant transcript expression of one genome over the other genome(s) vary from gene to gene [51]。In hexaploid wheat, a uniform level of expression for all the three homoeologues has been reported for approximately 20% of the unigene loci [52], whereas 20-29% genes did not express at least one homoeoallele [52,53]。Thus, the relative contribution of the three homoeologues ofTaSTOP1gene at transcript expression level was determined under Al and proton (H+) stresses in two bread wheat genotypes showing contrasting behaviour for Al toxicity. Transcript expression profiling ofTaSTOP1homoeologues in root and shoot tissues identified the predominance ofTaSTOP1-Ahomoeologue followed byTaSTOP1-DoverTaSTOP1-Bin root and only predominance ofTaSTOP1-Ain shoot tissues of both genotypes under control and stress (Al and pH) conditions (Figure6).Similarly, among the three homoeologues, the higher expression of one homoeologue of a MAD box transcription factor and Spa gene have also been observed in bread wheat [54,55]。Although, the presence ofciselements within the 5' untranslated region of a gene is unusual, it is not aberrant or abnormal [56]。Surprisingly, sequence analysis of 5' UTR ofTaSTOP1genes differentiated theTaSTOP1-Ahomoeologue fromTaSTOP1-BandTaSTOP1-Ddue to the presence of a pyrimidine-rich stretch and absence of light responsive element [Additional file4]。In tomato, the deletion of the 5' UTR containing pyrimidine-rich stretch from the HMG2 promoter reduced the level of HMG2 gene expression by a factor of 10 [56]。Therefore, among the three homogeologues in bread wheat, the up- or down-regulation of the expression of specific homoeologue could be the result from either the dominancy of ancestral diploid donor parent or early polyploidization-cis-regulatory variation.
Interestingly, the time-dependent Al-responsive expression of TaSTOP1 homoeologues observed in the root tissues of two bread wheat showing contrasting phenotypes for Al toxicity suggests a putative role for TaSTOP1 in Al resistance (Figure6A and B). Al responsiveSTOP1前女友pression has also been reported inArabidopsis[16], alfalfa [57] and common bean [58]。Similarly to Arabidopsis, the transcript expression of homoeologues ofTaSTOP1in the roots of diverse bread wheat genotypes was also modulated in response to proton stress [16]。It is noticeable that genotype Barbela 7/72/92 is highly resistant to Al toxicity compared with Anahuac, but in the absence of Al under low pH we did not observe significant differences between these genotypes for root growth [Additional file5]。It is worth noticing that the three homoeologues ofTaSTOP1have similar genomic structures, but showed a different transcript expression in response to Al and proton (H+) stress. These results may reveal that the homoeologues of TaSTOP1 are differentially contributing to Al or proton (H+) tolerance in bread wheat. Further work is in progress in order to definitely establish if these genes play a significant role in aluminium tolerance.
Conclusions
Bread wheat has wide genotypic variation for Al resistance [23,40] and the role of several chromosomes such as chromosome arms 2DL, 3DL, 4BL, 4DL, 6AL, 7AS and chromosome 7D, in Al tolerance has been revealed in classical genetic studies through chromosome manipulation in wheat [43]。Furthermore, several QTL associated with Al tolerance in bread wheat have also been reported by many researchers [22,24–26]。Contrarily to Arabidopsis and rice, due to the paucity ofa prioricandidate genes in wheat only two major QTL located on chromosome 4DL and 4BL have yet been elucidated at molecular level, showing the co-segregation with candidate genesTaALMT1homoeologue (4DL) andTaMATE1homoeologue (4BL), respectively [22,27]。使用染色体manipul古典研究ation as well as recent QTL mapping and genome-wide association analysis have also detected loci associated with Al resistance on homoeologous group 3 chromosomes (3A, 3B and 3D) in bread wheat [23–25,43]。Recently, the role of a zinc finger transcription factor ART1 identified through mutational analysis in rice has also been shown in natural variation of Al tolerance in rice, earlier which was suggested that it was not involved in Al tolerance [20,32]。
In the present investigation, we cloned and characterized the novel candidate genesTaSTOP1in bread wheat. The homoeologues ofTaSTOP1前女友hibited similar genomic structures, but showed biased transcript expression and different response to Al and proton (H+) toxicity. Furthermore,TaSTOP1-Bhomoeologue from Al tolerant genotypes Barbela 7/72/92 and Viloso Mole not only showed highest similarity but also contain the same SNP located in ZF4 domain than Al sensitive genotypes Anahuac, Chinese Spring and Saloio [Additional file2] [40]。Finally, in order to correlate with Al tolerance, it would be very interesting either to functionally characterize or further verify the role of TaSTOP1 because gene(s) underlying the QTL on homoeologous group 3 chromosomes particularly 3BL has not been so far identified in this important cereal.
Methods
Plant material and growth condition
The seedlings of selected bread wheat genotypes Barbela 7/72/92, Anahuac, Chinese Spring, Ruivo, Viloso Mole and Saloio classified in relation to Al tolerance [40] were grown in hydroponic solution (0.4 mM CaCl2, 0.65 mM KNO3, 0.25 mM MgCl2。6H2O, 0.1 mM (NH4)2SO4and 0.04 mM NH4NO3) and kept in controlled growth chamber under 14 h/26°C day and a 10 h/22°C night regime, with a light intensity of 150 μmol photons m-2s-1and a relative humidity of 65%.
Al stress assay
Four days old seedlings of Al resistant bread wheat genotype Barbela 7/72/92 and Al susceptible genotype Anahuac growing in hydroponic solution with pH 4.0 were shifted in fresh nutritive solution having 74 μM Al whereas control seedling were raised in only fresh hydroponic solution. For root re-growth measurement, after 24 h, roots were immersed in 0.1% eriochrome cyanine R (Sigma, Germany) dye solution for 10 minutes and prior to measurement; seedlings were allowed to grow in fresh nutritive solution for 48 h. For visual detection of Al accumulation in roots, hematoxylin assay was performed [59] and root samples were observed under microscope.
DNA and RNA extraction
Total genomic DNA was isolated from young leaf samples using the DNeasy Plant Mini Kit (Qiagen, Germany) and total RNA was extracted using Trizol method followed by purification using PureLink™ RNA Mini Kit (Ambion, Invitrogen, USA). The first-strand cDNA was synthesized in a final volume of 20 μl reaction containing: 1 μg RNA, 2 μl 10× RT buffer, 0.8 μl of 25× dNTP mix (100 mM), 2 μl of 10× RT random primers and 1 μl of Multiscribe reverse transcriptase (Applied Biosystems, USA).
Gene structure and cloning of full-length cDNA
TheArabidopsis thalianaAtSTOP1 (AT1G34370) protein sequence was retrieved from TAIR database (http://www.arabidopsis.org) and was used as the query sequence to search the GenBank wheat expressed sequence tag (EST) database. The homologous sequence ofSTOP1in wheat was obtained via tblastn in NCBI (http://www.ncbi.nlm.nih.gov).小麦EST同源克隆显示最高的sim卡ilarity withArabidopsis STOP1were retrieved.TaSTOP1(wheat EST) specific primers were designed to perform the sequential analysis in bread wheat genotype Barbela 7/72/92 [Additional file3]。Total RNA from the seedlings of Barbela 7/72/92 and Anahuac was employed to obtain the full-length cDNA ofTaSTOP1including 5’ UTR and 3’ UTR using Rapid Amplification of cDNA Ends (RACE) (SMARTer™ RACE cDNA Amplification Kit, clontech, USA). The genomic sequence ofTaSTOP1was obtained in six different bread wheat genotypes, namely Barbela 7/72/92, Anahuac, Ruivo, Viloso Mole, Saloio and Chinese Spring [Additional file6]。
Molecular analysis and construction of phylogenetic tree
The Pfam (http://www.sanger.ac.uk/Software/Pfam/search,shtml) software was used to identify potential domains and WOLF-PSORT (http://www.psort.org) was used to predict the intracellular localization of the TaSTOP1 protein. Fifty two STOP1 like protein sequences were collected from 32 different plant species including TaSTOP1 homoeologues from bread wheat genotype Barbela 7/72/92 using the Basic Local Alignment Search Tool (BLAST) programme from NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi) [Additional file7]。Phylogenetic tree was constructed by MEGA4 software using neighbor-joining method [60]。
Mapping ofTaSTOP1gene in wheat genome
All the nullitetrasomic and ditelosomic lines of Chinese Spring wheat except for the homoeologous group 2, 4 and 6 chromosomes used in present investigation were available in our lab, whereas remaining stocks were kindly provide by Prof. Adam J. Lukaszewski at Department of Botany and Plant Sciences, University of California, USA. On the basis ofTaSTOP1alignment, different pairs of specific primers for eachTaSTOP1homoeologue were designed [Additional file3]。TaSTOP1homoeologues (TaSTOP1-A,TaSTOP1-BandTaSTOP1-D) were amplified in the nullitetrasomic (N3AT3B, N3AT3D, N3BT3A, N3BT3D, N3DT3A and N3DT3B) and ditelosomic [Dt 3AL and 3AS (long and short arm), Dt 3BL and 3BS, Dt 3DL and 3DS] lines of Chinese Spring wheat.TaSTOP1homoeologues were assigned to the chromosomal arms based on the presence/absence of the PCR amplification products.
Physical localization ofTaSTOP1was performed byin situhybridization. Probe was prepared by PCR amplification of the 1.5 kb genomic region ofTaSTOP1from Barbela 7/72/92. Chromosome spreads from the root tips of Barbela 7/72/92 seedlings at mitotic metaphase, probe labelling and hybridization mixture were carried as described by Prietoet al.[61]。Probe labelling was confirmed by dot-blot and detection of hybridization signals was carried out using the Tyramide Signal Amplification Kit (TSA™, PerkinElmer Life and Analytical Sciences, Inc., Waltham, MA, USA). To identify wheat chromosomes with positive signals, samples were re-hybridized using simultaneously the pAS1 repetitive sequence and GAA-satellite sequence as probes [62,63]。The GAA-satellite sequence identifies all the A and B wheat chromosomes [62] whereas the pAs1 identifies chromosomes from the D genome [63]。Individual slides were observed under a Nikon Eclipse 80i microscope (Nikon Instruments Europe BV, UK). Images were captured with a Nikon CCD camera using the appropriate Nikon 3.0 software and processed with Photoshop 4.0 software (Adobe Systems Inc., San Jose, California, USA).
Transactivation assay
TaSTOP1was amplified by PCR from Barbela 7/72/92 using oligonucleotide primers containing restriction site forKpnI [Additional file3]。TheKpnI digestedTaSTOP1fragment was cloned in frame with thelexAgene in the plasmid YCp91 [31]。Transformation of the ligated products was performed in theEscherichia colistrainXL1-Blue recA1 endA1 gyrA96 thi-hsdR17 supE44 relA1 lac[F’proAB lacIqZDM15 Tn10(春节r)] (Stratagene, USA) and the positive clones were sequenced. The recombinant plasmid YCp91-TaSTOP1-Awas transformed in the yeast strain EGY48, which harbours the plasmid pSH18.84 encoding thelacZgene under the control of a promoter containing LexA – responsiveciselements. Transformants were grown in selective media and β-galactosidase measurements were performed as previously described [64,65]。
Analysis ofTaSTOP1前女友pression level
For Al assay, seedlings of two bread wheat genotypes Barbela 7/72/92 and Anahuac showing contrasting response to Al toxicity, were raised in hydroponic nutrient solution with pH 4.0 during four days and further, were shifted to fresh nutrient solution with (74 μM Al in the form of AlCl3·6H2O; stress treatment) or without Al (control treatment). Both root and shoot tissues were collected separately after treatment at specific time points (0, 2, 6, 12 and 24 h) from control and Al stress imposed seedlings. For pH assay, four days old seedlings of both genotypes grown in hydroponic nutritive solution with pH 6.0 were transferred in fresh nutritive solutions having different levels of pH (pH 6.0, pH 5.0 and pH 4.0). Root samples of both genotypes from each treatment were collected after 0 h and 6 h exposure to different levels of pH.
Three biological replicates of each sample were prepared and duplicate quantitative assays were performed for each cDNA sample.TaSTOP1homoeologues gene expression pattern was determined using the SYBR Premix Ex Taq (Takara, Japan) and the ABI 7500 Real-Time FAST PCR System (Applied Biosystems, USA). The 2-ΔΔCTmethod [66] was used to quantify the relative expression levels ofTaSTOP1homoeologues in comparison to18SRNAendogenous control.
Accession numbers
Sequence data from this article can be found in the GenBank database under the following accession numbers: Barbela 7/72/92 (TaSTOP1-A: GenBank number KF034793; TaSTOP1-B: GenBank number KF034794; TaSTOP1-D: GenBank number KF034795), Anahuac (TaSTOP1-A: GenBank number KF034796; TaSTOP1-B: GenBank number KF034797; TaSTOP1-D: GenBank number KF034798), Viloso Mole (TaSTOP1-A: GenBank number KF034801; TaSTOP1-B: GenBank number KF034802; TaSTOP1-D: GenBank number KF034803), Saloio (TaSTOP1-A: GenBank number KF034804; TaSTOP1-B: GenBank number KF034805; TaSTOP1-D: GenBank number KF034806), Chinese Spring (TaSTOP1-A: GenBank number KF034799; TaSTOP1-B: GenBank number KF034800) and Ruivo (TaSTOP1-D: GenBank number KF034807).
Abbreviations
- ALMT:
-
Aluminium-activated malate transporter
- MATE:
-
Multidrug and toxic compound exudation
- ALS:
-
Al-sensitive
- STAR:
-
Sensitive to Al rhizotoxicity
- Nrat1:
-
Nramp aluminum transporter 1
- STOP:
-
sensitive to proton rhizotoxicity
- ART:
-
Al resistance transcription factor
- QTL:
-
Quantitative trait loci/locus
- SNP:
-
Single nucleotide polymorphism
- ORF:
-
Open reading frame
- ZF:
-
Zinc finger domains
- FISH:
-
Fluorescentin situhybridization.
References
- 1.
von Uexküll HR, Mutert E: Global extent, development and economic impact of acid soils. Plant-Soil Interactions at Low pH: Principles and Management. Edited by: Date RA, Grundon NJ, Raymet GE, Probert ME. 1995, Dordrecht, The Neth: Kluwer Academic, 5-19.
- 2.
Kochian LV, Hoekenga OA, Piñeros MA: How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol. 2004, 55: 459-493. 10.1146/annurev.arplant.55.031903.141655.
- 3.
Simões CC, Melo JO, Magalhaes JV, Guimarães CT: Genetic and molecular mechanisms of aluminum tolerance in plants. Genet Mol Res. 2012, 11 (3): 1949-1957. 10.4238/2012.July.19.14.
- 4.
Ryan PR, Delhaize E, Jones DL: Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol. 2001, 52: 527-560. 10.1146/annurev.arplant.52.1.527.
- 5.
Ma JF: Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int Rev Cytol. 2007, 264: 225-252.
- 6.
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H: A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004, 37: 645-653. 10.1111/j.1365-313X.2003.01991.x.
- 7.
Magalhaes合资,刘J,吉马良斯CT,拉娜UG,阿尔维斯VM, Wang YH, Schaffert RE, Hoekenga OA, Pineros MA, Shaff JE, Klein PE, Carneiro NP, Coelho CM, Trick HN, Kochian LV: A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet. 2007, 39: 1156-1161. 10.1038/ng2074.
- 8.
Larsen PB, Geisler MJB, Jones CA, Williams KM, Cancel JD: ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J. 2005, 41: 353-363.
- 9.
Larsen PB, Cancel J, Rounds M, Ochoa V: Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta. 2007, 225: 1447-1458. 10.1007/s00425-006-0452-4.
- 10.
Huang CF, Yamaji N, Mitani N, Yano M, Nagamura Y, Ma JF: A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell. 2009, 21: 655-667. 10.1105/tpc.108.064543.
- 11.
Xia J, Yamaji N, Kasai T, Ma JF: Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci USA. 2010, 107: 18381-18385. 10.1073/pnas.1004949107.
- 12.
Stockinger EJ, Gilmour SJ, Thomashow MF:Arabidopsis thaliana CBF1encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA. 1997, 94: 1035-1040. 10.1073/pnas.94.3.1035.
- 13.
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K: Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998, 10: 1391-1406.
- 14.
Haake V, Cook D, Riechmann JL, Pineda O, Thomas MF, Zhang JZ: Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol. 2002, 130: 639-648. 10.1104/pp.006478.
- 15.
Yamaguchi-Shinozaki K, Shinozaki K: Transcriptional Regulatory Networks in Cellular Responses and Tolerance to Dehydration and Cold Stresses. Annu Rev Plant Biol. 2006, 57: 781-803. 10.1146/annurev.arplant.57.032905.105444.
- 16.
Iuchi年代,小山H, Iuchi,小林,Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M: Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci USA. 2007, 104: 9900-9905. 10.1073/pnas.0700117104.
- 17.
Yamaji N, Huang CF, Nagao S, Yano M, Sato Y, Nagamura Y, Ma JF: A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell. 2009, 21: 3339-3349. 10.1105/tpc.109.070771.
- 18.
Sawaki Y, Iuchi S, Kobayashi Y, Ikka T, Sakurai N, Fujita M, Shinozaki K, Shibata D, Kobayashi M, Koyama H: STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009, 150: 281-294. 10.1104/pp.108.134700.
- 19.
Liu JP, Magalhaes JV, Shaff J, Kochian LV: Aluminum activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57: 389-399. 10.1111/j.1365-313X.2008.03696.x.
- 20.
Famoso AN, Zhao K, Clark RT, Tung C-W, Wright MH, Bustamante C, Kochian LV, McCouch SR: Genetic Architecture of Aluminum Tolerance in Rice (Oryza sativa) Determined through Genome-Wide Association Analysis and QTL Mapping. PLoS Genet. 2011, 7 (8): e1002221-10.1371/journal.pgen.1002221. doi:10.1371/journal.pgen.1002221
- 21.
Century K, Reuber TL, Ratcliffe OJ: Regulating the regulators: The future prospects for transcription-factor-based agricultural biotechnology products. Plant Physiol. 2008, 147: 20-29. 10.1104/pp.108.117887.
- 22.
Raman H, Zhang KR, Cakir M, Appels R, Garvin DF, Maron LG, Kochian LV, Moroni JS, Raman R, Imtiaz M, Drake-Brockman F, Waters I, Martin P, Sasaki T, Yamamoto Y, Matsumoto H, Hebb DM, Delhaize E, Ryan PR: Molecular characterization and mapping of ALMT1, the aluminium-tolerance gene of bread wheat (Triticum aestivumL.). Genome. 2005, 48: 781-791. 10.1139/g05-054.
- 23.
Raman H, Stodart B, Ryan PR, Delhaize E, Emebiri L, Raman R, Coombes N, Milgate A: Genome-wide association analyses of common wheat (Triticum aestivuml .)种质alumini标识多个位点um resistance. Genome. 2010, 53 (11): 957-966. 10.1139/G10-058.
- 24.
Zhou LL, Bai GH, Ma HX, Carver BF: Quantitative trait loci for aluminum resistance in wheat. Mol Breed. 2007, 19 (2): 153-161. 10.1007/s11032-006-9054-x.
- 25.
Cai S, Bai GH, Zhang D: Quantitative trait loci for aluminum resistance in Chinese wheat landrace FSW. Theor Appl Genet. 2008, 117 (1): 49-56. 10.1007/s00122-008-0751-1.
- 26.
Navakode S, Weidner A, Lohwasser U, Röder MS, Börner A: Molecular mapping of quantitative trait loci (QTLs) controlling aluminium tolerance in bread wheat. Euphytica. 2009, 166 (2): 283-290. 10.1007/s10681-008-9845-8.
- 27.
Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E: A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol. 2009, 149: 340-351. 10.1104/pp.108.129155.
- 28.
Delhaize E, Ma JF, Ryan PR: Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 2012, 17 (6): 341-348. 10.1016/j.tplants.2012.02.008.
- 29.
Tovkach A, Ryan PR, Richardson AE, Lewis DC, Rathjen TM, Ramesh S, Tyerman SD, Delhaize E: Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiol. 2013, 161 (2): 880-892. 10.1104/pp.112.207142.
- 30.
Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K: WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007, 35 (2): W585-W587.
- 31.
Menezes RA, Amaral C, Delaunay A, Toledano M, Rodrigues-Pousada C: Yap8p activation inSaccharomyces cerevisiaeunder arsenic conditions. FEBS Lett. 2004, 566 (1): 141-146.
- 32.
Ryan PR, Tyerman SD, Sasaki T, Furuichi T, Yamamoto Y, Zhang WH, Delhaize E: The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. J Exp Bot. 2011, 62 (1): 9-20. 10.1093/jxb/erq272.
- 33.
Hoekenga OA, Maron LG, Pineros MA, Cancado GMA, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T, Matsumoto H, Yamamoto Y, Koyama H, Kochian LV: AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci USA. 2006, 103: 9738-9743. 10.1073/pnas.0602868103.
- 34.
Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF: An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007, 48: 1081-1091. 10.1093/pcp/pcm091.
- 35.
Maron LG, Pineros MA, Guimarães CT, Magalhaes JV, Pleiman JK, Mao C, Shaff J, Belicuas SN, Kochian LV: Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J. 2010, 61: 728-740. 10.1111/j.1365-313X.2009.04103.x.
- 36.
Collins NC, Shirley NJ, Saeed M, Pallotta M, Gustafson JP: An ALMT1 gene cluster controlling aluminum tolerance at the Alt4 locus of rye (Secale cerealeL.). Genetics. 2008, 179: 669-692. 10.1534/genetics.107.083451.
- 37.
Yokosho K, Yamaji N, Ma JF: Isolation and characterization of two MATE genes in rye. Func Plant Biol. 2010, 37: 296-303. 10.1071/FP09265.
- 38.
Barceló J, Poschenrieder C: Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review. Environ Exp Bot. 2002, 48: 75-92. 10.1016/S0098-8472(02)00013-8.
- 39.
Poschenrieder C, Gunse B, Corrales I, Barceló J: A glance into aluminum toxicity and resistance in plants. Sci Total Environ. 2008, 400: 356-368. 10.1016/j.scitotenv.2008.06.003.
- 40.
Martins-Lopes P, Maçãs B, Guedes-Pinto H: Portuguese bread wheat germplasm evaluation for aluminium tolerance. Cereal Res Commun. 2009, 37 (2): 179-188. 10.1556/CRC.37.2009.2.4.
- 41.
Wolfe SA, Nekludova L, Pabo CO: DNA recognition by Cys(2)His(2) zinc finger proteins. Annu Rev Biophys Biomol Struct. 2000, 29: 183-212. 10.1146/annurev.biophys.29.1.183.
- 42.
Huang S, Sirikhachornkit A, Su XJ, Faris J, Gill B, Haselkorn R, Gornicki P: Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proc Natl Acad Sci USA. 2002, 99 (12): 8133-8138. 10.1073/pnas.072223799.
- 43.
Aniol A, Gustafson JP: Chromosome location of genes controlling aluminum tolerance in wheat, rye, and triticale. Can J Genet Cytol. 1984, 26: 701-705.
- 44.
Clarke BC, Phongkham T, Gianibelli MC, Beasley H, Bekes F: The characterisation and mapping of a family of LMW-gliadin genes: Effects on dough properties and bread volume. Theor Appl Genet. 2003, 106: 629-635.
- 45.
Danyluk J,凯恩NA,布列塔尼人G,正规AE,福勒DB,Sarhan F: TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiol. 2003, 132: 1849-1860. 10.1104/pp.103.023523.
- 46.
Watanabe N, Koval SF: Mapping of chlorina mutant genes on the long arm of homologous group 7 chromosomes in common wheat with partial deletion lines. Euphytica. 2003, 129: 259-265. 10.1023/A:1022276724354.
- 47.
Pérez R, de Bustos A, Jouve N, Cuadrado A: Localization ofRad50,a single-copy gene, on group 5 chromosomes of wheat, using a FISH protocol employing Tyramide for Signal Amplification (Tyr-FISH). Cytogenet Genome Res. 2009, 125: 321-328. 10.1159/000235938.
- 48.
Cabrera A, Martin A, Barro F:In-situcomparative mapping (ISCM) of Glu-1 loci inTriticumandHordeum。Chromosome Res. 2002, 10: 49-54. 10.1023/A:1014270227360.
- 49.
Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-Shinozaki K: Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 2004, 136: 2734-2746. 10.1104/pp.104.046599.
- 50.
Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H: Transposase-derived transcription factors regulate light signalling in Arabidopsis. Science. 2007, 318: 1302-1305. 10.1126/science.1146281.
- 51.
Feldman M, Levy AA, Fahima T, Korol A: Genomic asymmetry in allopolyploid plants: wheat as a model. J Exp Bot. 2012, 63 (14): 5045-5059. 10.1093/jxb/ers192.
- 52.
Mochida K, Yamazaki Y, Ogihara Y: Discrimination of homoeologous gene expression in hexaploid wheat by SNP analysis of contigs grouped from a large number of expressed sequence tags. Mol Genet Genomics. 2004, 270: 371-377. 10.1007/s00438-003-0939-7.
- 53.
Bottley A, Xia GM, Koebner RMD: Homoeologous gene silencing in hexaploid wheat. Plant J. 2006, 47: 897-906. 10.1111/j.1365-313X.2006.02841.x.
- 54.
Shitsukawa N, Tahira C, Kassai K, Hirabayashi C, Shimizu T, Takumi S, Mochida K, Kawaura K, Ogihara Y, Murai K: Genetic and epigenetic alteration among three homoeologous genes of a class E MADS box gene in hexaploid wheat. Plant Cell. 2007, 19: 1723-1737. 10.1105/tpc.107.051813.
- 55.
Ravel C, Martre P, Romeuf I, Dardevet M, El-Malki R, Bordes J, Duchateau N, Brunel D, Balfourier F, Charmet G: Nucleotide polymorphism in the wheat transcriptional activator Spa influences its pattern of expression and has pleiotropic effects on grain protein composition, dough visco elasticity, and grain hardness. Plant Physiol. 2009, 151: 2133-2144. 10.1104/pp.109.146076.
- 56.
Daraselia ND, Tarchevskaya S, Narita JO: The promoter for tomato 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Gene 2 has unusual regulatory elements that direct high-leve1 expression. Plant Physiol. 1996, 112: 727-733. 10.1104/pp.112.2.727.
- 57.
Chen Q, Zhang XD, Wang SS, Wang QF, Wang GQ, Nian HJ, Li KZ, Yu YX, Chen LM: Transcriptional and physiological changes of alfalfa in response to aluminium stress. J Agric Sci. 2011, 149: 737-751. 10.1017/S0021859611000256.
- 58.
Eticha D, Zahn M, Bremer M, Yang ZB, Rangel A, Rao IM, Horst W: Transcriptomic analysis reveals differential gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Ann Bot. 2010, 105: 1119-1128. 10.1093/aob/mcq049.
- 59.
Polle E, Konzak CF, Kittrick JA: Visual detection of aluminium tolerance levels in wheat by hematoxylin staining of seedlings roots. Crop Sci. 1978, 18: 823-827. 10.2135/cropsci1978.0011183X001800050035x.
- 60.
Tamura K, Dudley J, Nei M, Kumar S: MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007, 24: 1596-1599. 10.1093/molbev/msm092.
- 61.
Prieto P, Ramirez MC, Ballesteros J, Cabrera A: Identification of intergenomic translocations involving wheat, Hordeum vulgare and Hordeum chilense chromosomes by FISH. Hereditas. 2001, 135: 171-174.
- 62.
Pedersen C, Rasmussen SK, Linde-Laursen I: Genome and chromosome identification in cultivated barley and related species of the Triticeae (Poaceae) by in situ hybridization with the GAA-satellite sequence. Genome. 1996, 39: 93-104. 10.1139/g96-013.
- 63.
Rayburn AL, Gill BS: Isolation of a D-genome specific repeated DNA sequence from Aegilops squarrosa. Plant Mol Biol Rep. 1986, 4: 102-109. 10.1007/BF02732107.
- 64.
Miller JH: Experiments in Molecular Genetics: Assay of β-Galactosidase. 1972, NY: CSH Laboratory Press, Cold Spring Harbor, 352-355.
- 65.
Azevedo D, Nascimento L, Labarre J, Toledano MB, Rodrigues-Pousada C: TheS. cerevisiaeYap1 and Yap2 transcription factors share a common cadmium-sensing domain. FEBS Lett. 2007, 581 (2): 187-195. 10.1016/j.febslet.2006.11.083.
- 66.
Livak KJ, Schmittgen TD: Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT。Method. 2001, 25 (4): 402-408. 10.1006/meth.2001.1262.
Acknowledgements
We thank Fundação para a Ciência e Tecnologia (FCT) of Portugal for providing grants to ALGO (SFRH/BD/45556/2008) and RAM (SFRH/BPD/26506/2006) and PML (PEst-OE/EQB/LA0023/2011). We thank Dr. Renato Rodrigues-Pousada (L’Aquila University, Italy) and anonymous reviewers for valuable comments on manuscript.
Author information
Affiliations
Corresponding authors
Additional information
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
ALGO performed most of the experiments and drafted the manuscript; PP helped inin-situhybridization assay; RAM helped in transactivation assay and CB guided for cloning, chromosomal localization and figure preparation, CRP, HGP and PML coordinated the experiments and helped in finalizing the manuscript. Finally, all authors read and approved the final manuscript.
Electronic supplementary material
Multiple alignments of the homoeologues of
Additional file 1:TaSTOP1in bread wheat genotype Barbela 7/72/92.(PNG 555 KB)
Phylogenetic tree and multiple alignments of
Additional file 2:TaSTOP1homoeologues in different species of wheat including some bread wheat genotypes.(DOC 114 KB)
Alignment of homoeologues of
Additional file 4:TaSTOP15UTR region.(RTF 41 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Garcia-Oliveira, A.L., Benito, C., Prieto, P.et al.Molecular characterization ofTaSTOP1homoeologues and their response to aluminium and proton (H+) toxicity in bread wheat (Triticum aestivumL.).BMC Plant Biol13,134 (2013). https://doi.org/10.1186/1471-2229-13-134
Received:
Accepted:
Published:
Keywords
- Aluminium
- TaSTOP1
- Triticum aestivum L
- In situhybridization
- Transcription factor
- Transactivation
- Homoeologue
- pH