- Research article
- Open Access
- Published:
The Arabidopsis LRR-RLK,PXC1, is a regulator of secondary wall formation correlated with the TDIF-PXY/TDR-WOX4 signaling pathway
BMC Plant Biologyvolume13, Article number:94(2013)
Abstract
Background
Although a number of leucine-rich repeat receptor-like kinase-encoding genes (LRR-RLKs) have been identified in plants, a functional role has been determined for only a few. Recent studies have demonstrated that an LRR-RLK, PXY/TDR, is important for the process of secondary vascular development. Other studies have indicated that PXY/TDR is unlikely to be the sole LRR-RLK involved in this complex process.
Results
In this study,in silicoanalyses led to the identification of threeArabidopsis LRR-RLKgenes (PXY-correlated; PXC1, 2, 3) with transcript accumulation profiles that correlated strongly with several key regulators of vascular development, includingPXY/TDR,HB-8,REV, andCLE41. Expression profiling using qPCR and promoter:reporter lines indicated that all threePXCgenes are associated with the vasculature. One in particular,PXC1(At2g36570),had a strong correlation withPXY/TDR. Shiftingpxc1mutants from long-days to short-days showed that loss of the gene led to a dramatic reduction in secondary wall formation in xylem fibers. Transcript analysis of mutants for a variety of secondary cell wall-associated genes, includingPXY/TDRindicated that the pathways mediated by PXC1 connect with those mediated by the TDIF-PXY/TDR-WOX4 system.
Conclusions
数据表明,LRR-RLK PXC1 involved in secondary cell wall formation in xylem fibers. Whereas further study is needed to identify the ligands and mode of action of the PXC1 protein, it is clear from this work that similarly to the shoot apical meristem (SAM), secondary vascular development requires contributions from a number of LRR-RLKs.
Background
LRR-RLKs (leucine-rich repeat receptor-like kinases) comprise the largest group within the RLK (receptor-like kinase) superfamily in plants. Among the more than 400RLKgenes identified in theArabidopsisgenome, over half areLRR-RLKs[1]。TheLRR-RLKscan be grouped into 13 subfamilies (I to XIII) [2]。Although the functions of most LRR-RLKs remain undiscovered, it has been suggested that plant LRR-RLKs can be divided into two broad functional categories [2]。That is, some appear to function in plant growth and developmental processes such as morphogenesis, organogenesis and hormone signaling, while others appear to be primarily involved in mediating responses to biotic or abiotic stresses and therefore can be said to be defense-related. Some LRR-RLKs have been demonstrated to possess dual functions, either through signaling pathway cross-talk or due to their ability to recognize multiple ligands [3]。Well known examples of LRR-RLKs involved in the regulation of plant growth and development are the CLV3 (CLAVATA3)-CLV1 (CLAVATA1)-WUS (WUSCHEL) signaling system in the SAM (Shoot Apical Meristem) and the similar, CLE40 (CLAVATA3/ESR40)-CLV1-ACR4 (CRINKLY4)-WOX5 (WUSCHEL RELATED HOMEOBOX 5) signaling system in the RAM (Root Apical Meristem) [4–9]。In the SAM, the CLV1 LRR-RLK is essential for maintaining a balance between stem cell division and differentiation, and therefore, growth control in the shoot [6,10,11]。Loss of CLV1 leads to the accumulation of undifferentiated cells in the SAM. Three other LRR-RLKs that operate in the SAM, the BAM (BARELY ANY MERISTEM) proteins, form a monophyletic group with CLV1, are also involved in maintaining meristem function. Although CLV1 and the BAMs both operate in maintaining SAM function, their expression profiles and functions differ. In contrast to CLV1, the loss of BAM function leads to a reduction in the number of undifferentiated cells [12]。Clearly, LRR-RLKs fulfill multiple roles in complex processes such as meristem function.
LRR-RLKs have now been shown to be involved in all three major plant meristems, the SAM and RAM, and the vascular cambium, that produces cells for secondary vascular development. InArabidopsis,信号系统组成的一个小CLE peptide, the TDIF (TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR), and its receptor PXY/TDR (TDIF RECEPTOR/ PHLOEM INTERCALATED WITH XYLEM) regulates the behavior of vascular stem cells [13]。Genetic analyses showed that at least two pathways diverge early in TDIF-PXY/TDR signaling and the WOX4 (WUSCHEL RELATED HOMEOBOX 4), which belongs to the WUS subclade in the WOX family [14], is required for promoting the proliferation of procambial/cambial stem cells but not for repressing their commitment to xylem differentiation in response to the TDIF signal [15]。Correct spatial separation of the expression of the genes encoding PXY/TDR and TDIF, is essential for generating the spatial cues necessary for ordered secondary vascular development[16]。
As the only well described pathway so far, the TDIF-PXY/TDR signal transduction pathway has been suggested to be required both very early in vascular development to orientate the polarity of the vascular bundle, and continuously throughout development to regulate the process [16]。Ectopic expression of TDIF-related genes results in pleiotropic phenotypes including a bushy appearance with small leaves [17,18]。Recently, TDIF and CLE42 peptide were found to have anin vivoactivity to enhance axillary bud formation and there are indications that PXY/TDR is involved in this process [19]。Together, these results indicate that the TDIF ligand-PXY/TDR signal transduction pathway is an important regulator of multiple developmental processes [19]。
Given the size of the LRR-RLK gene family and the evidence of multiple active LRR-RLKs in the SAM, it is likely that other multiple LRR-RLKs are involved in the complex process of secondary vascular development. In this work, we aimed to identify LRR-RLKs other than PXY/TDR that contribute to secondary vascular development through interactions, direct or indirect, with the TDIF-PXY/TDR pathway. Given the importance of the localization of gene expression for PXY/TDR function, we initially performed anin silicoco-expression and functional clustering analyses. ThreeLRR-RLKs(At2g36570, At5g01890 and At2g41820) were identified that had similar transcript profiles toPXY/TDR. We named themPXCforPXY/TDR-correlatedgenes. Evidence from loss-of-function and gain-of-function analyses showed thatPXC1in particular plays a TDIF-PXY/TDR associated role in the process of secondary cell formation in fiber cells.
Results and discussion
Co-expression profiling and functional clustering analyses identified threeAtLRR-RLKs与PXY/TDR
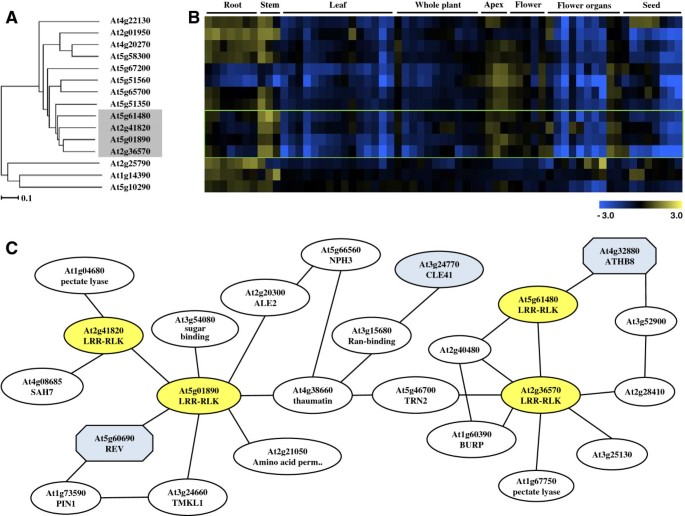
In order to develop our understanding of the functions of AtLRR-RLKs in vascular development, a hierarchical cluster analyses using the microarray data in the Genevestigator database was performed for allAtLRR-RLKs[20]。The dendrogram in Additional file1was used to assess transcript profile similarities between the genes. Six out of the 7 genes that clustered withPXY/TDRexhibited preferential expression in the vasculature, with the highest transcript levels occuring in the stem, apex and floral organs (Figure1A,1B). Of the 7,MOL1(At5g51350, MORE LATERAL GROWTH1),RUL1(At5g05160, REDUCED IN LATERAL GROWTH1) andVH1(At2g01950, VASCULAR HIGHWAY1) have already been reported for their vascular functions [21,22]。The other three,At2g41820,At5g01890andAt2g36570are largely uncharacterised. We named the genes asPXC PXY/TDR-correlatedgenes,PXC1(At2g36570),PXC2(At5g01890), andPXC3(At2g41820). TAIR database information of these three genes indicated that they may be broadly functional. For example,PXC1displayed a decreased expression level in theArabidopsisleaves treated by salt [23]andPXC2appear to be down-regulated inArabidopsisseedlings under anoxia [24]。ForPXC3, its transcription was dramatically repressed inArabidopsiscell suspensions upon salicylic acid treatment [25]。In an investigation into the roles of LRR-RLKs inArabidopsisroot development, germination of a line mutated inPXC1, N634974耐盐(200米M NaCl) and osmotic stress. Apxc2mutant allele was found to be sensitive to darkness and resistant to osmotic stress treatment (400 mM mannitol) [1]。在一起,结果显示的作用Arabidopsis PXCgenes in defense and other stress-related responses. A putative soybean ortholog of PXC1,GmLRK1gene has been studied in some detail [26]。MutatingGmLRK1led to reduced lignification in leaf cells and defective leaf cell elongation. The authors hypothesized that GmLRK1is is involved in the regulation of cell expansion by influencing the development of cell wall architecture [26]。
Phylogeny ofAtLRR-RLKfamily members and their coexpression patterns.(A)Part of the expressional phylogenetic tree of someAtLRR-RLKgenes;(B)Digital northern heat map representation ofAtLRR-RLKgenes among different tissue types;(C)The co-expressed gene network using thePXC1/2/3andPXYas query genes on the ATTED-II platform of Tokyo University.
In order to further explore the possibility of a role for the threePXCgenes in vasculature development, we developed a putative gene co-regulation network using the ATTED-II suite of programs (based on publicly available microarray data of 58 experiments, 1388 slides collected by AtGenExpress) [27,28](Additional file2). The analyses led to the identification of a putative relationship betweenPXY/TDRandPXC1(Figure1C). The homeobox transcription factor,AtHB8(HOMEOBOX GENE 8), that is regarded as a procambium and protoxylem cell identity marker, appeared to be similarly associated withPXY/TDRandPXC1[29]。PXC1also correlated strongly with genes encoding enzymes involved in the cell expansion process (Figure1C). ThePXC2 gene correlated withPXC3 and withREVOLUTA(REVAt5g60690).AtHB8andREVare Class III HD-ZIP transcriptional regulators and both play important roles in vascular differentiation [30]。CLE41, that encodes the peptide ligand for the PXY/TDR receptor [31], was located betweenPXC1andPXC2in the coexpression network (Figure1C).The bioinformatic data, therefore, indicated connections between PXY/TDR, and the three PXC proteins in vascular development.
Expression patterns ofPXYand threePXCgenes in vascular tissues
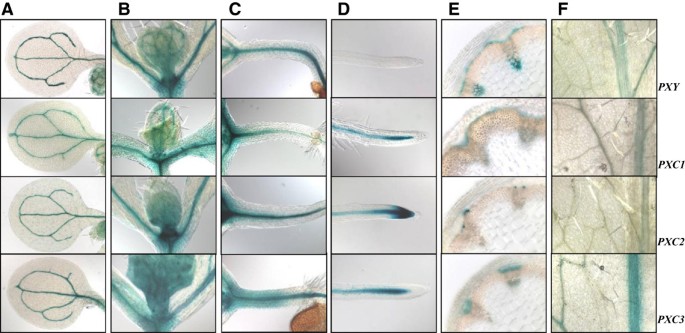
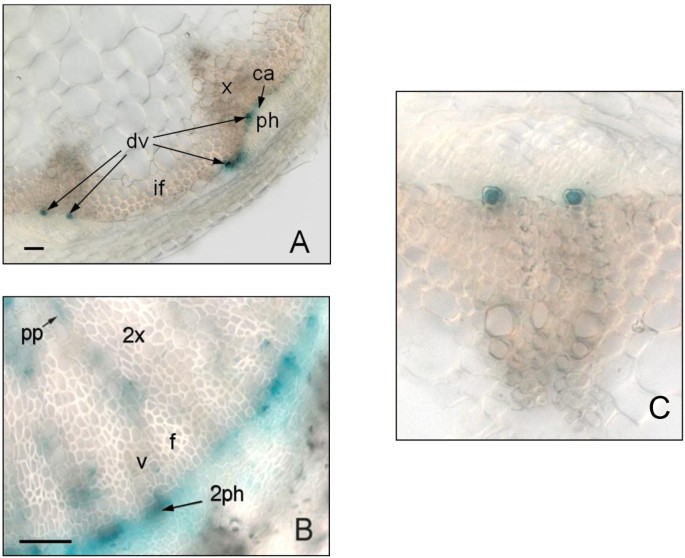
In order to further explore the associations betweenPXC1/2/3andPXY/TDR, we examined their respective expression patterns using a native promoter-driven GUS (β-glucuronidase) reporter system.PXY/TDRhas been reported to be expressed in the vasculature of a variety of organs including leaves, roots and the stem [16]。Its expression has been shown to be confined to the procambial cells in the developing vascular bundles [16]。The pattern of GUS activity observed in thepPXY::GUSwas similar to the pattern oberseved in thepPXC1::GUS, pPXC2::GUS,andpPXC3::GUSlines. Specifically, GUS was observed primarily in the vascular strands in cotyledons, the shoot apex, hypocotyls, roots and leaves (Figure2A-2D,2F). In the inflorescence stems of thepPXY::GUSline, GUS was observed in the protoxylem, procambial cells and in the interfascicular cambial regions (Figure2E). In thepPXC1::GUSline,GUS staining was observed primarily in the developing xylem of both inflorescence stem and hypocotyl (Figure2E, Figure3B).PXC1expression overlapped withPXYexpression in the basal stem, except that the staining was very faint in protoxylem (Figure2E). InpPXC2::GUSplants, GUS staining in the inflorescence stem was primarily observed in the differentiating vessel cells (Figure2E, Figure3A,3C). The GUS staining pattern in the secondary vasculature of thepPXC3::GUSplants was similar to that ofpPXY::GUS, except that inPXC3::GUS, no GUS was not expressed in the interfascicular region (Figure2E). The GUS staining of the four lines diverged in the roots. GUS was observed in the root tip in thepPXC2::GUSline, close to the quiescent center in thepPXC1::GUSandpPXC3::GUS行和elongation zone in thepPXY::GUSline (Figure2D) [13,15,16]。In contrast to the similarities in the GUS staining patterns in the vascular tissues, there was little similarity between the GUS outside these tissues (i.e. in floral tissues) (Additional file3).
Validation of the microarray data for differential gene expression by GUS staining of transgenic plants harboringpPXY::GUS, pPXC1::GUS,pPXC2::GUS and pPXC3::GUS.One-week-old plants for a-d, five-week-old plants for e-h and the floral stem was about 15 cm tall.(A)GUS staining in the cotyledons;(B)GUS staining in the shoot apex;(C)GUS staining in the hypocotyls;(D)GUS staining in the root tip;(E)GUS staining in the stem cross section;(F)GUS staining in the leaves.
Expression levels ofPXC1in vascular tissues
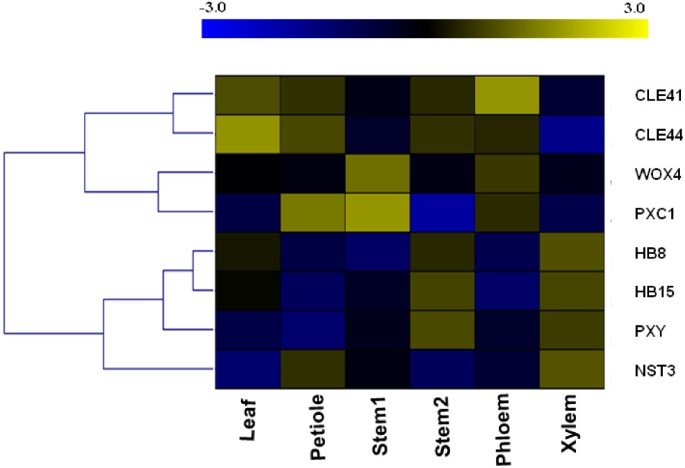
Because the strongest predicted links were betweenPXC1andPXY/TDR, we concentrated our efforts on this gene. To further investigate the function ofPXC1in vascular development, transcript levels ofPXC1were determined in parallel with seven known regulators of vascular development in different plant tissues including the leaf lamina (as a control), petiole, young inflorescence stem, old inflorescence stem and hypocotyl. Our data showed that the transcripts forPXYwere most abundant in the xylem fraction of hypocotyls, with levels increasing in older inflorescence stems (Additional file4C). Expression patterns forHB8,HB15(HOMEOBOX GENE 15) andNST3(NAC TRANSCRIPTION FACTOR3) were similar to those observed forPXY(Additional file4A, B and D).HB8andHB15are both recognized as molecular markers of procambial cells [32,33]andNST3encodes a known regulator of secondary cell wall formation in xylem fibers [34,35]。Interestingly, the qRT-PCR data also indicated a similar expression pattern betweenPXC1andWOX4, with the highest level of transcript observed in young stem (Additional file4G and H).CLE41andCLE44both encode the B-type CLE peptides that act as the ligands for the TDIF-PXY/TDR signal transduction pathway [31]。As previously reported, theCLE41transcript was found to be mainly associated with the phloem fraction (Additional file4E) [13]。This fraction may, however, include some cambium cells as a result of the sample collection (peeling) method used [13]。CLE44transcript appeared more evenly distributed among the tissue fractions, with a significantly lower level of transcripts in the xylem tissue (Additional file4F), which is consistent with the recent report of phloem-specific expression ofCLE44::GUS[36]。To summarize the expression patterns described above, a heat map was generated using the qPCR data. Strikingly, the heat map highlighted the similarities betweenPXC1andWOX4in vascular tissues (Figure4).
A heat-map (log transformed) of qRT-PCR gene expression data for main regulators of vascular development in 5-week old wild-type Arabidopsis.Stem1 denoted the base of main inflorescence stem 10 cm in height above the uppermost rosette leaf and stem2 denoted the base of main inflorescence stem 30 cm in height above the uppermost rosette leaf. Phloem and xylem were ontained by peeling method.
Loss ofPXC1function suppresses secondary cell wall formation in xylem fibers
ThePXC1gene encodes a predicted protein containing a putative kinase domain and an extracellular domain with 21 leucine-rich repeats (eLRR). The predicted PXC1 belongs to the LRR-RLK subfamily III [2,37]along with CLV1 (27% amino acid identity) and PXY/TDR (28% amino acid identity). Protein motif search in the Pfam and SMART databases predicted that the kinase domain of PXC1 resembles that of the animal receptor tyrosine kinase domain, which is an unusual characteristic for a plant RLK [26]。Phosphorylation of the soybean homologue of PXC1 (GmLRK1) has been demonstrated to be induced by plant protein extracts, which suggested that some plant proteins may interact with GmLRK1 and phosphorylate itin vivo[26]。Based on the high degree of sequence divergence between PXC1 and other subfamilies of LRR-RLKs, no firm prediction can be made as to its functional role.
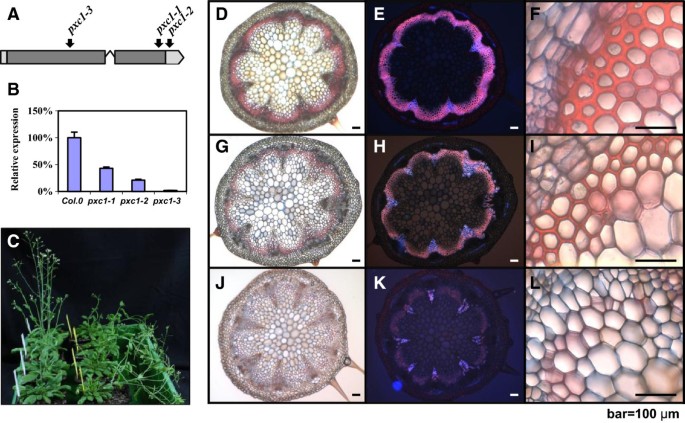
Three T-DNA insertion lines were identified forPXC1in the NASC (the European Arabidopsis Stock Center) mutant collection, including two SALK lines and one WiscDsLox T-DNA line. These three alleles were designated aspxc1-1 (SALK_134974),pxc1-2 (SALK_134975), andpxc1-3 (WiscDsLox470G6), respectively. Bothpxc1-1andpxc1-3contain insertions in the coding sequence, whilepxc1-2has a T-DNA inserted in the 3'-UTR (Figure5A). Results of qPCR analyses using a gene specific primer set located in the 3’-UTR indicated that the level ofPXC1mRNA in three mutants waspxc1-1>pxc1-2>pxc1-3(Figure5B).pxc1-3displayed only background level ofPXC1expression, suggesting that this allele might represent a null mutant (Figure5B). Several aspects of plant morphology were affected by the mutation ofPXC1. The inflorescence stems of thepxc1mutants were taller than those of the wild-type, with the phenotype most pronounced in thepxc1-3line (Additional file5). By contrast, the inflorescence stem ofpxymutant plants were shorter than the wild-type [16]。When grown under long-day conditions,pxc1mutants did not show significant difference from the wild-type in terms of cellular morphology as seen from transverse sections of the inflorescence stem (data not shown). Secondary vascular development is enhanced in the inflorescence stems ofArabidopsiswhen plants are grown under long day conditions (16/8 day/night) and transferred to short-day conditions (8/16 day/night) immediately after bolting. In both wild-type andpxc1-2plants, the inflorescence stem grew vertically from this point. In contrast, the stems ofpxc1-1andpxc1-3plants were unable to support the weight of the continued upright growth of the stem (Figure5C). Ligin staining in cross sections taken from the base ofpxc1-1,pxc1-3and wild-type inflorescent stems indicated that the two mutant lines were defective in vascular lignification (Figure5D-L), which then provided an explanation to the inability of the inflorescence stem to support an upright growth. Tissue polarity in thepxc1mutants appeared to be retained, as opposed to the polarity phenotype in thepxymutant (Figure5G-J). Secondary cell wall thicking in fiber cells was also considerably reduced inpxc1-1plants and absent frompxc1-3plants (Figure5I, 5L), indicating a reduced capacity for secondary cell wall synthesis and lignifications in thepxc1mutants (Figure5H, 5K). The vascular bundles of the long day grown plants and those shifted from long to short days wer compared by close-ups in Figure6. Interestingly,PXC1has been identified in the repertoire of genes regulated bySND2, which is an indirect target of a principal regulator of fiber secondary cell wall formation, SND1 [38,39]。Overexpression ofSND2produced a fiber cell-specific increase in secondary cell wall thickness inArabidopsisstems andPXC1was slightly up-regulated in this transgenic line [38]。Thus, the reduced cell wall thickness in the interfascicular fiber cell ofpxc1-3andpxc1-1mutants indicated that PXC1 is likely playing a role in secondary cell wall formation of fiber cells.
Characterization of threepxc1mutants.(A)Location of insertions creatingpxc1mutant alleles. The location of the insertion site is indicated. Lines indicate non-coding sequence and boxes indicate coding sequence;(B)mRNA expression levels inpxc1-1,pxc2-1, andpxc1-3plants were determined by the real-time PCR.EF1αwere used as an internal control;(C)pxc1-1andpxc1-3mutant plants are pendent in phenotype under short-day conditions. Photographs of wild-type (Col0) are on the left and those ofpxc1-3are on the right. Arabidopsis plants taken 51 days after germination (DAG);(D-L)Phloroglucinol staining for lignin in the cross-sections taken from the base of 30 cm wild-type(D-F),pxc1-1(G-I), andpxc1-3(J-L). Scale bars represent 100μm in f, i, l close-up.
Phloroglucinol staining for lignin in the cross-sections taken from 30 cm above the base of wild-typeandpxc1-3before and after long-day to short-day transition.(A)Wild-typeArabidopsisplant grown in long-day conditions;(B)Arabidopsis pxc1-3plant grown in long-day conditions;(C)Wild-typeArabidopsisplant grown in short-day conditions after bolting;(D)Arabidopsis pxc1-3plant grown in short-day conditions after bolting.
The interactions betweenPXC1andTDIF-PXY/TDRsignaling pathway
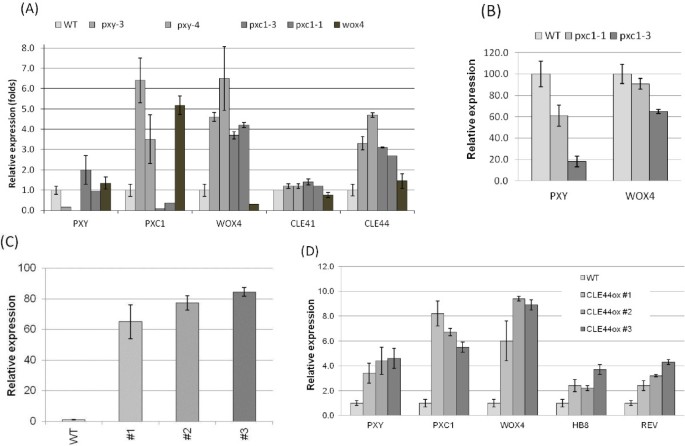
qPCR analysis ofPXY,PXC1,CLE41,CLE44andWOX4expression was analyzed in thepxy/tdr,pxc1,cle41,cle44andwox4mutants. Transcript abundances ofPXYandCLE41were not dramatically affected in thepxy/tdr,pxc1andwox4knockout lines (Figure7A). The transcript level ofPXC1, WOX4andCLE44were significantly increased inpxy,pxc1andwox4mutants compared to the wild-type (Figure7A). The elevated expression level ofWOX4in thepxybackground was unexpected since thatWOX4is a key downstream target of the TDIF-PXY/TDR signaling pathway [13]。This result indicated a possibility that PXY might not be the only receptor acting upstream of WOX4. Meanwhile, instead of young seedlings [13], 5-week-old hypocotyls, which contain more secondary growth, were used in this study and the implication of PXY/TDR in xylem development has not been investigated in details. The dramatic increase inPXC1transcripts occurred in thepxyandwox4knockout mutants indicated that either the PXY-WOX4 pathway negatively regulate the expression ofPXC1, or thatPXC1is upregulated in these mutants as a compensatory measure (Figure7A). These data also suggested that the signal transduction pathways mediated by PXY and PXC1 are at least partly overlapping. However, it is hard to specify the position of PXC1 relative to the TDIF/PXY/WOX4 pathway because we could not judge which alterations in gene expression resulting from forward responses or feedback responses.
Transcript level changes of genes involved in TDIF/PXY/WOX4 pathway and vascular development markers in wild-typeArabidopsisand various mutants and overexpressors.(A)Gene expression levels analyzed by qPCR in the hypocotyls of 5-week-old wild-typeArabidopsisand mutants under long-day conditions;(B)Change in gene expression level in the basal stem part of two 51-day-oldPXC1mutants after the long-day to short-day shifting experiment;(C)RelativeCLE44expression level measured by qRT-PCR in Col-0 and threeCLE44 oxplants with inflorescence stems as tall as 15 cm;(D)Relative expression level of marker genes in wild-type and threeCLE44 oxlines with inflorescence stems as tall as 15 cm.
As opposed to the expression profiles of plants grown under long-day conditions, the short-day shift experiment induced a strong decrease inPXY/TDRandWOX4transcript levels in the inflorescence stems of thepxc1mutants, particularly in thepxc1-3line (Figure7B). The reduced capacity to produce an interfascicular cambium in thepxc1mutants may explain the reduction inPXY/TDRandWOX4transcript levels, or vice versa, the reduced expression ofPXY/TDRandWOX4may be the underlying cause of the lack of interfascicular cambium. Future research will attempt to answer this question. It has been reported that the exogenous application of the TDIF/CLE41/44 peptide ligand resulted in an increase in the transcript levels ofHB8,HB15,WOX4andPXYgenes [40]。We similarly over-expressedCLE44inArabidopsisand analyzed its effects onPXC1transcript levels. ThreeCLE44oxlines exhibiting from 65 to 85-fold increases inCLE44transcript levels compared to wild-type level were identified and analyzed (Figure7C). These lines exhibited similar phenotypes to theCLE41oxandCLE42oxlines reported previously [13,31]。Transcripts forPXC1,PXY/TDR,WOX4,HB-8andREVwere elevated compared to wild-type plants in all three of ourCLE44oxlines (Figure7D). Once again, the explanation may be that the changes in gene expression are the result of developmental abnormalities. That is to say that the affected genes might not be directly regulated byCLE44over-expression, but the result of changes in cellular composition, such as an increase in the abundance of dividing/undifferentiated cells in the transgenic line (Figure7D). However, the similarities betweenPXC1and elements in theTDIF-PXY/TDRpathway indicate that PXC1 functions synergistically with the TDIF-PXY/TDR signaling pathway.
Conclusions
LRR-RLK receptors have been shown to mediate multiple signal transduction pathways. It is clear from work in the SAM that the combined actions of multiple LRR-RLKs, each with defined functions, are required to maintain the balance between stem cell division and differentiation in meristems. In vascular tissue, TDIF-PXY/TDR signal transduction pathway plays multiple roles in xylem development including the promotion of cambial cell division and repression of xylogenesis [31]。In this work, fromin silicoanalyses, a new LRR-RLK component involved in the regulation of plant vasculature development, PXC1, was introduced with its expression patterns correlated to that ofPXYgene. PXC1 probably plays its roles in a regulatory network which also incorporates the PXY/TDR-WOX4 signaling pathway and regulates the maturation of interfascicular fiber cells. The co-regulation network suggested that the loss ofPXC1function might retard the initiation of secondary cell wall deposition by prolonging the course of cell wall remodeling and reorganization during the procedure of cell expansion.
Materials and methods
Expression profiling, co-expression analyses and gene functional clustering
Microarray expression data sets were explored for the predictedAtLRR-RLKgenes using the Arabidopsis Affymetrix GeneChip® average data available on the GENEVESTIGATOR analysis tool site (http://www.genevestigator.ethz.ch) [20]。The Gene Co-expression Analysis (GeneCAT) Toolbox athttp://genecat.mpg.de/cgi-bin/Ainitiator.py[41]was used to generate the Expression Tree which clustered genes by the similarity of their expression profiles and visualized those similarities using a dendogram. To minimize the effects of experimental artifacts, data were renormalized, and Pearson’s correlation coefficient between genes was weighted in ATTED-II [28]。
Identification of loss-of-function mutants and construction of transgenic Arabidopsis
Seeds for segregating T3 plants harboring thepxc1alleles in the Col-0 ecotype background were obtained from the NASC (Nottingham Arabidopsis Stock Centre. The location of the T-DNA insertion was determined by sequencing and homozygous lines were identified by PCR. For the production of the35Spro::CLE44construct, the coding sequence of Arabidopsis CLE44 was amplified from genomic DNA, cloned into pDONR201 vector (Invitrogen), and subcloned into the pK2GW7 vector using the Gateway cloning system (Invitrogen). Nativepromoter::GUS-GFPfusion constructs were made forPXYandPXC1/2/3by cloning the amplified promoter regions into the binary vector pKGWFS7™ [42]viapDONR201 (Invitrogen). Vectors were then transformed intoAgrobacterium tumefaciensstrain GV3101 (pMP90). Arabidopsis plants were transformed using the floral dip method [44]。Positive transgenic Arabidopsis plants were selected based on kanamycin resistance conferred by the T-DNA. T2 seeds from at least 24 independent positive lines for each construct were harvested for expression analyses.
Histochemistry
Plant tissues at various developmental stages were vacuum-infiltrated for 2 min in GUS solution including 1 mM X-gluc, 50 mM sodium phosphate (pH 7.0), 0.1% Triton X-100, 1 mM potassium ferricyanide and 1 mM potassium ferrocyanide, and incubated at 37°C overnight. Destaining of the samples were performed by incubation in 0.24 M HCl and 20% MeOH solution at 55°C for 15 min, then in 7% NaOH and 60% EtOH solution at room temperature for 15 min. The samples were dehydrated through ethanol series (40%, 20%, and 10% in water). For sectioning, samples were embedded in 4% (w/v) agar and sectioned at 50μm with a vibratome (Leica vt1000s). Samples were then mounted in glycerol and analyzed by bright field transmitted microscopy using an Axioplan 2 microscope (Carl Zeiss Inc. Thornwood, NY, USA). Images were captured by AxioCam HRc and Axiovision software (AxionVs40 V4.5.0.0).
Safranin/alcian-blue staining
Plants were grown under long day conditions and then were moved into short day conditions right after bolting. Long day conditions were 16h light/8h dark, 75% humidity, 150μE of irradiance and short day conditions were 8 h light/16 h dark, 75% humidity, 150μE of irradiance. For sectioning, samples were embedded in 4% (w/v) agar, and sectioned at 50μm with a vibratome (Leica vt1000s). Sections were then stained in one part safranin (1% w/v safranin in 50% ethanol) and two parts alcian-blue (1% v/w alcian-blue, 1% v/v formalin, 36% formaldehyde) in 0.05% glacial acetic acid. The sections were subsequently rinsed in ddH2O and mounted in 50% glycerol. Slide-mounted sections were viewed using a Zeiss Axioplan 2 compound microscope with a Zeiss AxioCamHRc digital camera (Carl Zeiss, Inc., Thornwood, NY, USA).
Gene expression analyses by quantitative PCR
All samples came from five-week-old wild type plants under long-day conditions when the inflorescence stems reached a height of 25 cm, except that stem1 was from younger plants whose inflorescence was 10 cm high. Fully expanded leaves were harvested without the midrib, and petioles were harvested from the fully expanded leaves. Stem1 (younger stem) and stem2 (older stem) were both from the basal part of the inflorescence stems at 1–5 cm above the rosette. All nodes were removed from the stem samples. The phloem and xylem samples were obtained simply by separating the bark/phloem from the xylem core. Total RNA (0.2μg) was used for cDNA synthesis using the Thermoscript RT-PCR kit (Invitrogen Life Technologies, USA). Real-time qPCR was performed with the LightCycler instrument (Roche Diagnostics, Germany). Each mRNA value was corrected by the measurements obtained in the same sample for 18S mRNA and elongation factor 1 (EF1α) using Delta Delta method [44]。The primer sequences utilized in this study were listed in Additional file6. Each amplification included three technical replicates and their results were averaged to give the value for a single biological replicate. Three biological replicates were prepared for each treatment using material harvested from 10–15 plants in each case. The means of relative expression for each sample were examined using one-way analysis of variance (ANOVA) method (significance atP< 0.05).
Author’s contributions
JW, MK, LZ, PC and DD performed the experimental work. BZ, ON and GS designed and coordinated the project. JW, BZ and BJ wrote the paper. All authors read and approved the final manuscript.
References
Ten Hove CA, Bochdanovits Z, Jansweijer VM, Koning FG, Berke L, Sanchez-Perez GF, Scheres B, Heidstra R: Probing the roles of LRR RLK genes inArabidopsis thalianaroots using a custom T-DNA insertion set. Plant Mol Biol. 2011, 76 (1–2): 69-83.
Shiu SH, Bleecker AB: Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA. 2001, 98 (19): 10763-10768.
Afzal AJ, Wood AJ, Lightfoot DA: Plant receptor-like serine threonine kinases: roles in signaling and plant defense. Mol Plant Microbe Interact. 2008, 21 (5): 507-517.
Clark SE, Running MP, Meyerowitz EM: CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development. 1993, 119 (2): 397-418.
Clark SE, Williams RW, Meyerowitz EM: The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997, 89: 575-585.
Schoof H, Lenhard M, Haecker A, Mayer KFX, Jurgens G, Laux T: The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000, 100 (6): 635-644.
品牌U, Grunewald M, Hobe M,Simon R: Regulation of CLV3 expression by two homeobox genes in Arabidopsis. Plant Physiol. 2002, 129 (2): 565-575.
Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T: Conserved factors regulate signalling inArabidopsis thalianashoot and root stem cell organizers. Nature. 2007, 446 (7137): 811-814.
Stahl Y, Wink RH, Ingram GC, Simon R: A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr Biol. 2009, 19 (11): 909-914.
Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R: Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science. 2000, 289 (5479): 617-619.
Mayer KFX, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T: Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998, 95 (6): 805-815.
DeYoung BJ, Bickle KL, Schrage KJ, Muskett P, Patel K, Clark SE: The CLAVATA1-related BAM1, BAM2 and BAM3 receptor kinase-like proteins are required for meristem function in Arabidopsis. Plant J. 2006, 45 (1): 1-16.
Hirakawa Y, Shinohara H, Kondo Y, Inoue A, Nakanomyo I, Ogawa M, Sawa S, Ohashi-Ito K, Matsubayashi Y, Fukuda H: Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc Natl Acad Sci USA. 2008, 105 (39): 15208-15213.
Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T: Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev Cell. 2008, 14 (6): 867-876.
Hirakawa Y, Kondo Y, Fukuda H: TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell. 2010, 22 (8): 2618-2629.
Fisher K, Turner S: PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr Biol. 2007, 17 (12): 1061-1066.
Strabala TJ, O'Donnell PJ, Smit AM, Ampomah-Dwamena C, Martin EJ, Netzler N, Nieuwenhuizen NJ, Quinn BD, Foote HC, Hudson KR: Gain-of-function phenotypes of many CLAVATA3/ESR genes, including four new family members, correlate with tandem variations in the conserved CLAVATA3/ESR domain. Plant Physiol. 2006, 140 (4): 1331-1344.
Whitford R, Fernandez A, De Groodt R, Ortega E, Hilson P: Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells. Proc Natl Acad Sci USA. 2008, 105 (47): 18625-18630.
Yaginuma H, Hirakawa Y, Kondo Y, Ohashi-Ito K, Fukuda H: A novel function of TDIF-related peptides: promotion of axillary bud formation. Plant Cell Physiol. 2011, 52 (8): 1354-1364.
Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W: GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004, 136 (1): 2621-2632.
Agusti J, Lichtenberger R, Schwarz M, Nehlin L, Greb T: Characterization of transcriptome remodeling during cambium formation identifies MOL1 and RUL1 as opposing regulators of secondary growth. PLoS Genet. 2011, 7 (2): e1001312.
Clay NK, Nelson T: VH1, a provascular cell-specific receptor kinase that influences leaf cell patterns in Arabidopsis. Plant Cell. 2002, 14 (11): 2707-2722.
Sottosanto JB, Gelli A, Blumwald E: DNA array analyses ofArabidopsis thalianalacking a vacuolar Na+/H+ antiporter: impact of AtNHX1 on gene expression. Plant J. 2004, 40 (5): 752-771.
Loreti E, Poggi A, Novi G, Alpi A, Perata P: A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiol. 2005, 137 (3): 1130-1138.
Krinke O, Ruelland E, Valentova O, Vergnolle C, Renou JP, Taconnat L, Flemr M, Burketova L, Zachowski A: Phosphatidylinositol 4-kinase activation is an early response to salicylic acid in Arabidopsis suspension cells. Plant Physiol. 2007, 144 (3): 1347-1359.
Kim S, Kim SJ, Shin YJ, Kang JH, Kim MR, Nam KH, Lee MS, Lee SH, Kim YH, Hong SK, et al: An atypical soybean leucine-rich repeat receptor-like kinase, GmLRK1, may be involved in the regulation of cell elongation. Planta. 2009, 229 (4): 811-821.
Obayashi T, Kinoshita K, Nakai K, Shibaoka M, Hayashi S, Saeki M, Shibata D, Saito K, Ohta H: ATTED-II: a database of co-expressed genes and cis elements for identifying co-regulated gene groups in Arabidopsis. Nucleic Acids Res. 2007, 35(Database issue): D863-D869.
Obayashi T, Hayashi S, Saeki M, Ohta H, Kinoshita K: ATTED-II provides coexpressed gene networks for Arabidopsis. Nucleic Acids Res. 2009, 37(Database issue): D987-D991.
Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, Morelli G: The expression of the Athb-8 homeobox gene is restricted to provascular cells inArabidopsis thaliana. Development. 1995, 121 (12): 4171-4182.
Otsuga D, DeGuzman B, Prigge MJ, Drews GN, Clark SE: REVOLUTA regulates meristem initiation at lateral positions. Plant J. 2001, 25 (2): 223-236.
Etchells JP, Turner SR: The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development. 2010, 137 (5): 767-774.
Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, Morelli G: The expression of theATHB-8同源框基因受到限制provascular cells inArabidopsis thaliana. Development. 1995, 121: 4171-4182.
Ohashi-Ito K, Fukuda H: HD-zip III homeobox genes that include a novel member, ZeHB-13 (Zinnia)/ATHB-15 (Arabidopsis), are involved in procambium and xylem cell differentiation. Plant Cell Physiol. 2003, 44 (12): 1350-1358.
Mitsuda N, Iwase A, Yamamoto H, Yoshida M, Seki M, Shinozaki K, Ohme-Takagi M: NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell. 2007, 19 (1): 270-280.
Mitsuda N, Ohme-Takagi M: NAC transcription factors NST1 and NST3 regulate pod shattering in a partially redundant manner by promoting secondary wall formation after the establishment of tissue identity. Plant J. 2008, 56 (5): 768-778.
Malinowski R, Smith JA, Fleming AJ, Scholes JD, Rolfe SA: Gall formation in clubroot-infected Arabidopsis results from an increase in existing meristematic activities of the host but is not essential for the completion of the pathogen life cycle. Plant J. 2012, 71 (2): 226-238.
萧若元SH, Karlowski WM,潘R, Tzeng YH,梅耶尔KF,Li WH: Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004, 16 (5): 1220-1234.
Hussey SG, Mizrachi E, Spokevicius AV, Bossinger G, Berger DK, Myburg AA: SND2, a NAC transcription factor gene, regulates genes involved in secondary cell wall development in Arabidopsis fibres and increases fibre cell area in Eucalyptus. BMC Plant Biol. 2011, 11: 173.
Zhong R, Demura T, Ye ZH: SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell. 2006, 18 (11): 3158-3170.
Hirakawa Y, Kondo Y, Fukuda H: Regulation of vascular development by CLE peptide-receptor systems. J Integr Plant Biol. 2010, 52 (1): 8-16.
Mutwil M, Obro J, Willats WGT, Persson S: GeneCAT - novel webtools that combine BLAST and co-expression analyses. Nucleic Acids Res. 2008, 36: W320-W326.
Karimi M, Inze D, Depicker A: GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002, 7 (5): 193-195.
Clough SJ, Bent AF: Floral dip: a simplified method for Agrobacterium-mediated transformation ofArabidopsis thaliana. Plant J. 1998, 16 (6): 735-743.
Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29 (9): e45.
Acknowledgements
这项研究是由国家项目上客y Basic Research Project (2012CB114500), National High Technology Research and Development Program (2011AA100200) and National Natural Science Foundation (31270644, 31170564 and 31070600) of China. It was also supported by Swedish Foundation for Strategic Research.
Author information
Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Electronic supplementary material
12870_2013_1307_MOESM4_ESM.pdf
Additional file 4:Transcript levels of main regulators of vascular development in wild-typeArabidopsis.Stem1 denoted the main inflorescence stem 10 cm in height above the uppermost rosette leaf and stem2 denoted the main inflorescence stem 30 cm in height above the uppermost rosette leaf. Phloem and xylem were ontained by peeling method. (PDF 74 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open AccessThis article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wang, J., Kucukoglu, M., Zhang, L.et al.The Arabidopsis LRR-RLK,PXC1, is a regulator of secondary wall formation correlated with the TDIF-PXY/TDR-WOX4 signaling pathway.BMC Plant Biol13,94 (2013). https://doi.org/10.1186/1471-2229-13-94
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/1471-2229-13-94
Keywords
- LRR-RLK
- Arabidopsis
- Secondary Wall Formation
- TDIF-PXY/TDR-WOX4 Signaling