- Research article
- Open Access
- Published:
Functional analysis of the Landsbergerectaallele ofFRIGIDA
BMC Plant Biologyvolume14, Article number:218(2014)
Abstract
Background
Most of the natural variation in flowering time inArabidopsis thalianacan be attributed to allelic variation at the geneFRIGIDA(FRI,AT4G00650), which activates expression of the floral repressorFLOWERING LOCUS C(FLC, AT5G10140). Usually, late-flowering accessions carry functionalFRIalleles (FRI-wt), whereas early flowering accessions contain non-functional alleles. The two most frequent alleles found in early flowering accessions are the ones present in the commonly used lab strains Columbia (FRI-Col) and Landsbergerecta(FRI-Ler), which contain a premature stop codon and a deletion of the start codon respectively.
Results
Analysis of flowering time data from various Arabidopsis natural accessions indicated that theFRI-Lerallele retains some functionality. We generated transgenic lines carrying theFRI-ColorFRI-Lerallele in order to compare their effect on flowering time, vernalization response andFLCexpression in the same genetic background. We characterize their modes of regulation through allele-specific expression and their relevance in nature through re-analysis of published datasets. We demonstrate that theFRI-Lerallele inducesFLCexpression, delays flowering time and confers sensitivity to vernalization in contrast to the true nullFRI-Colallele. Nevertheless, theFRI-Lerallele revealed a weaker effect when compared to the fully functionalFRI-wtallele, mainly due to reduced expression.
Conclusions
The present study defines for the first time the existence of a new class of Arabidopsis accessions with an intermediate phenotype between slow and rapid cycling types. Although using available data from a common garden experiment we cannot observe fitness differences between accessions carrying theFRI-Leror theFRI-Colallele, the phenotypic changes observed in the lab suggest that variation in these alleles could play a role in adaptation to specific natural environments.
Background
As plants are sessile organisms, adaptation to the environment is essential for their survival and reproductive success. Mechanisms regulating the response to environmental cues enable a proper timing of key events in a plant's life. One crucial event, resulting from the integration of endogenous and environmental signals, is the switch from vegetative to reproductive development. The annual speciesArabidopsis thalianaoccurs in the northern hemisphere in a broad range of latitudes differing substantially in day length, temperature and other ecological factors [1].As a result of adaptation to specific habitats, Arabidopsis accessions have evolved two main life history strategies. Winter-annuals germinate in autumn, survive winter as a rosette and flower in the following summer, whereas summer-annuals germinate in spring or summer and finish their reproduction cycle in the same year. Variation between these distinct strategies has been associated with allelic variation at the genesFRIGIDA(FRI) andFLOWERING LOCUS C(FLC), which act epistatically to regulate flowering time [2],[3].Arabidopsis individuals containing functional alleles at these two loci flower very late or not at all, unless they receive a prolonged exposure to cold (vernalization).
FLCencodes a MADS-box transcription factor that binds to the promoters of floral initiators such asFLOWERING LOCUS T (FT)andSUPPRESSOR OF CONSTANS OVEREXPRESSION 1 (SOC1)to repress their transcription. Flowering occurs whenFLCis downregulated by proteins of the vernalization and/or autonomous pathways, reviewed in [4]-[7].Transcriptional activation ofFLCrequires integrated activity of diverse chromatin remodeling and histone-modifying complexes, reviewed in [8].When Arabidopsis plants are vernalized, expression ofFLCis decreased and maintained at reduced level by different epigenetic marks in a Polycomb-mediated process involving long non-coding RNAs [9]-[11].The epigenetic silencing ofFLCis quantitatively modulated and underlies Arabidopsis natural variation for vernalization response [12]-[14].Two main haplogroups ofFLChave been defined mainly by polymorphisms within the first intron, an important region to maintain silencing induced by vernalization [15],[16].These haplogroups were shown to underlie differences in flowering time among natural accessions of Arabidopsis, but only when a functionalFRIallele is present [15].
Despite the central role ofFLC, most of the variation in flowering time has been found to correlate with natural allelic diversity atFRI[3].FRIis the founding member of a family of seven Arabidopsis proteins that contain two coiled-coil domains and show no homology to other proteins [2],[17].在order to regulate flowering time,FRIbuilds the scaffold protein of a transcription activator complex that mediates diverse chromatin modifications atFLC[18],[19].Furthermore,FRIis suggested to be involved in co-transcriptional processes that link the function of 5′ end capping with transcription and efficient splicing ofFLC[20].Vernalization abolishes the effect ofFRIand silencesFLCas described above [9],[12].
A considerable number of differentFRIhaplotypes have been identified within accessions from a wide range of latitudes [14],[21],[22] or more restricted geographic regions [23],[24].Studies on these alleles led to the conclusion that early flowering types evolved by multiple independent mutational events from winter-annuals containing an ancestral functionalFRIallele (FRI-wt) [25].Two distinct deletions inFRIare believed to confer early flowering in most of the rapid cycling accessions. The Columbia allele (FRI-Col) carries a 16 bp-deletion resulting in a premature stop codon and, thus, a truncated protein missing a part of the C-terminal [2],[14].But the most frequent deleteriousFRImutation in nature is a 376 bp-deletion combined with a 31 bp-insertion in the promoter as observed in Landsbergerecta(FRI-Ler) [14],[21],[22].This mutation disrupts the translational start but, due to a second alternative start codon, a short out-of-frame protein might be built [2].The loss-of-functionFRIalleles found in Lerand Col are widely used as examples of positive selection towards rapid cycling accessions [26],[27].
Although theFRI-LerandFRI-Colalleles are always classified as a single non-functional group, there are evidences for differences in their effects. First, accessions carrying the Ler-type deletion but not the Col-type deletion show considerable variation in flowering time andFLClevels [14],[28].Then, several studies find variation in flowering time associated with the chromosomal region ofFRIin mapping populations derived from accessions containing theFRI-Lerallele crossed to accessions with a loss-of-function allele [28]-[30].在all these cases, the Lerallele was associated with a delay in flowering time. This delay was attributed to additional loci in the same region asFRI, although these have never been identified.
This article provides the first robust evidence that the Lerallele ofFRIis functional. In contrast to the true nullFRI-Colallele, theFRI-Lerallele is able to induceFLCexpression, resulting in delayed flowering and increased vernalization sensitivity. Nevertheless, theFRI-Lerallele has a weaker effect than the fully functionalFRI-wtallele. The reduced functionality of theFRI-Lerallele mainly results from its lower expression putatively due to cis regulatory polymorphisms in its promoter. Our finding defines a new functional class ofFRIalleles, in which accessions carrying theFRI-Lerallele would flower in between the late and early flowering groups defined so far.
Results
Effects of theFRI-LerandFRI-Colalleles on flowering time and vernalization response
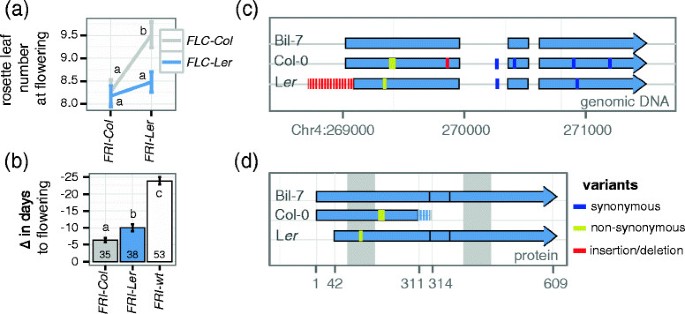
We investigated if theFRIalleles present in Col-0 and Lershow evidences of different functionality by analyzing flowering time data from a recombinant inbred line (RIL) population derived from a cross between the two accessions [31].Because the effect ofFRIon flowering time depends on the alleles atFLC, we took into account the genotype of the RILs at both loci [15].While Col-0 harbors a functionalFLCallele, Lerhas been described to contain a weak allele with a transposon-like insertion in the first intron [25],[32],[33].As shown in Figure1a, plants carrying theFRI-Lerallele flower significantly later than plants carrying theFRI-Colallele, although only in the presence of the strongFLCallele of Col-0 (two-way ANOVA,FRIp = 0.0095,FLCp = 0.0138, interaction p = 0.0824). This epistatic interaction suggests the existence of a functionalFRIallele in Ler, as no other gene located in this region of chromosome 4 has been shown to delay flowering time through interactions with a locus in the region of chromosome 5 containingFLC.
Functional comparison of the most common deleteriousFRIalleles. (a)Leaf number at flowering from individuals in the Col x Lerrecombinant inbred line set grouped by their genotype atFRIandFLC.(b)Vernalization response of Arabidopsis natural accessions measured as the reduction of days to flowering in plants vernalized for 5 weeks at 4°C compared to unvernalized plants. Accessions are grouped by theirFRIalleles as described in [22].The number at the bottom of each bar indicates the number of accessions in each group; error bars in(a)and(b)correspond to the standard error of the mean. Letters in each bar or point represent significance groups as determined by Tukey HSD test.(c)Exonic organization forFRI-wt(Bil-7),FRI-ColandFRI-Ler.Red regions indicate the indels characteristic forFRI-ColandFRI-Ler.(d)Predicted proteins for all three alleles shown in(c). The dashed region inFRI-Colrepresents a shift in the ORF caused by the 16 bp deletion. Shaded regions in the background indicate predicted coiled-coil domains.
Because strong vernalization requirements are associated with functionalFRIalleles, we decided to analyze variation in vernalization sensitivity among Arabidopsis accessions [34],[35].We calculated the decrease in flowering time in response to vernalization in a published dataset containing 126 Arabidopsis accessions classified asFRI-Col, FRI-LerorFRI-wt[22].Accessions carrying theFRI-Lerallele accelerated flowering by 10 days in response to vernalization (Figure1b). This acceleration was intermediate to the low response of accessions containing theFRI-Coldeletion and the high response of accessions carrying theFRI-wtallele (Figure1b). The same trend was found for the vernalization response quantified as total leaf number, although the difference between accessions carrying theFRI-Lerallele and theFRI-Colallele was not statistically significant (Additional file1).
Taken together, our results disagree with the common belief that theFRIallele present in Leris not functional. However, from this analysis we cannot completely rule out the possibility that the observed phenotypes are caused by additional loci in linkage disequilibrium withFRI.
Structural characterization of theFRI-LerandFRI-Colalleles
We looked for further evidences of a functionalFRI-Lerallele by studying sequence variation among Arabidopsis accessions. We sequenced genomic DNA fromFRIincluding its upstream and downstream regions in Col-0, Ler-1 and Bil-7, the latter being a winter-annual accession that contains a functionalFRI-wtallele identical to the published H51 allele [2],[14].All polymorphisms found inFRI-ColandFRI-Lercorrespond exactly to the ones described before, such as the insertion/deletions that define these two allelic classes (Figure1c; [2],[14]). The H51 allele is predicted to encode a fully functional 609 aa protein [GenBank: AAG23415] while the Col allele yields a 314 aa protein [GenBank: AEE81913], truncated due to a premature stop codon resulting from a 16 bp deletion in exon 1 (Figure1c and d; [2]). In the case ofFRI-Ler, a 376 bp deletion combined with a 31 bp insertion removes the translational start, but creates a new, out-of-frame start codon that is predicted to yield a 41 aa protein [2].在terestingly, in addition to the out-of-frame start codon,FRI-Lercontains an in-frame ATG codon downstream of the original start, which would result in a protein missing 42 aa of the N-terminus. Apart from this deletion and one conservative amino acid change from L to I in the first coiled-coil domain, the FRI-Lerprotein is identical to the functional Bil-7 allele (Figure1d). There are no known functional motifs in the deleted segment of the FRI protein, and, thus, if transcription and translation occurred from the downstream in-frame start codon, the resulting FRI-Lerprotein could possibly be as functional as the full-length Bil-7 protein.
We looked for evidences of transcription of this longFRI-Lerallele using RNA-seq reads from seven accessions containing the characteristic Lerindel [36].We found signals of expression across the full length of the gene, including the C-terminal part, in all accessions (see Additional file2). This raised the question of whether this longFRI-Lertranscript is translated into a protein and if so, whether it is sufficient to delay flowering and to confer vernalization sensitivity.
Comparing the effect of theFRI-Lerversus theFRI-Colallele in transgenics
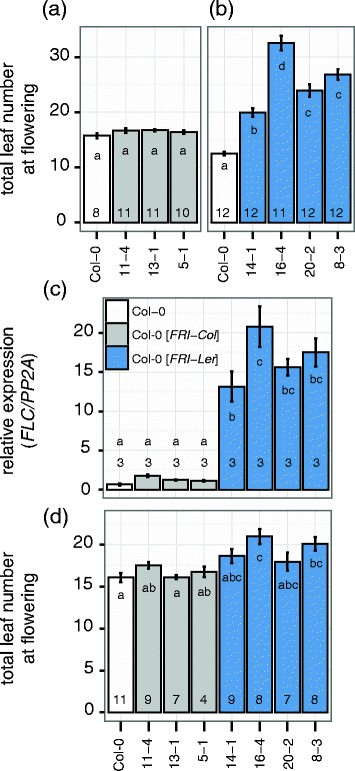
We studied the existence of a functionalFRI-Lerallele by comparing its function with that of theFRI-Colallele in transgenic plants. For this, we cloned the putative coding region plus the upstream and downstream region of both alleles and transformed them in a common background. We chose Col-0 as a recipient because our previous results suggest thatFRI-Colcarries a loss-of-function mutation (Figure1), and because Col-0 has been shown to contain a strongFLCallele that is activated in the presence of aFRI-wtallele [3].First, we confirmed the lack of function of theFRI-Colallele by growing three independent T3lines homozygous for theFRI-Coltransgene (Col-0[FRI-Col在温室])在漫长的一天的条件下。在this experiment none of the transgenic lines was significantly different from wild type (Figure2a). In a consecutive experiment in similar conditions we grew homozygous T3transgenic lines containing theFRI-Lertransgene (Col-0[FRI-Ler]) and observed a significant delay in flowering in all lines compared with the Col-0 wild type (Figure2b), confirmingFRI-Lerfunctionality.
Characterization of transgenic lines carrying theFRI-ColorFRI-Lerallele. (a)Flowering time expressed as total leaf number (rosette + cauline) from homozygous T3包含一个单独的插入行FRI-Coltransgene are shown in comparison to the untransformed Col-0 wild type.(b)Experiment as in(a)but using homozygous T3single insertion lines transformed withFRI-Ler.(c)Expression ofFLCfor the same genotypes as above. In this experiment, leaf tissue was collected from 10 day-old seedlings grown in long day conditions. The number in each bar indicates the number of biological replicates used. Expression was normalized to the expression ofPP2A.(d)Flowering time after vernalization quantified as total leaf number for the same lines as above. Plants were vernalized for four weeks at 4°C and subsequently grown under long day conditions. The number in each bar indicates the number of individual plants per line analyzed. Error bars represent the standard error of the mean. Letters in each bar represent significance groups as determined by Tukey HSD test.
BecauseFRIdelays flowering time through upregulation of the floral repressorFLC[3], we performed qRT-PCR to test the expression ofFLCin the transgenic plants. As expected, we found elevated expression ofFLCassociated with theFRI-Lertransgene but not with theFRI-Col transgene (Figure2c), despite all lines presenting increasedFRIexpression (Additional file3). In addition, a prolonged cold treatment, known to abolishFLCexpression, significantly reduced leaf number in Col-0[FRI-Ler] lines, but not in Col-0 wild type or in Col-0[FRI-Col] lines (p < 0.001, p = 0.06 and p = 0.28 for the interaction between vernalization and genotype in a two-way ANOVA respectively, compare Figure2d and Figure2a and b).
Altogether, our results demonstrate that theFRI-Lerallele, but not theFRI-Colallele, has the ability to delay flowering,and that this delay is achieved through upregulation ofFLC.
Expression ofFRIandFLCin natural accessions
The functionality of theFRI-Lerallele is contrasting with its frequent presence among early flowering accessions [2],[14].Our analyses also show that, although accessions carryingFRI-Leralleles have stronger vernalization responses than accessions containingFRI-Colalleles, they do not reach the levels observed in lines carryingFRI-wtalleles (Figure1b and Additional file1). This suggests a reduced functionality of theFRI-Lerallele in comparison with theFRI-wtallele, which can be caused by differences in the protein sequence, in expression levels or in post-transcriptional/post-translational mechanisms. Previous works have pointed to the low expression of theFRI-Lerallele, possibly as a consequence of the indel in its promoter [2],[37].This led us to focus on a possible transcription inhibition as the cause for early flowering in natural accessions containing this allele.
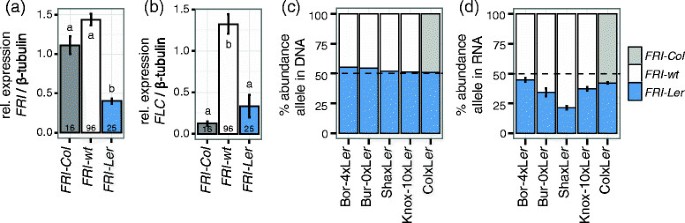
We analyzed the expression profiles ofFRIand its downstream targetFLCin 137 Arabidopsis accessions containingFRI-Ler,FRI-ColorFRI-wtalleles [38].Accessions carryingFRI-Leralleles showed significantly reduced expression ofFRIwhen compared to accessions carryingFRI-ColorFRI-wtalleles, suggesting a regulatory defect in theFRI-Lerallele (Figure3a). Interestingly, this residualFRI-Lerexpression results in higherFLClevels than in the accessions carryingFRI-Colalleles, although this difference is not significant (Tukey HSD test p value p = 0.079), possibly due to the large variation inFLCexpression found among accessions withFRI-Leralleles (compare error bars in Figure3b).
Relative expression levels ofFRIandFLCin Arabidopsis accessions and F1hybrids grouped by theirFRIallele. (a and b)137 Arabidopsis accessions were grown in long days under greenhouse conditions, sampled after four weeks and expression levels ofFLCandFRIwere quantified from northern hybridizations [38].Letters in each bar indicate the significance groups as determined by a Tukey HSD test. The number in each bar indicates the number of accessions in each group.(c and d)Positions with SNPs specific to Ler-1 or Col-0 were targeted using pyrosequencing in genomic DNA(c)or in cDNA(d)of F1hybrids. Leaf material for hybrids involving Bor-4, Bur-0, Knox-10 or Sha was collected at the time of bolting from plants growing under 12-hour photoperiods in an environmental chamber. Leaf material from hybrids involving Col-0 was collected from 10 day-old seedlings grown in the greenhouse in long days. Error bars indicate the standard error of the mean.
Allele-specific expression ofFRI-LerandFRI-Col
There are two alternative causes for the lowerFRIexpression found in Arabidopsis accessions carrying theFRI-Lerallele when compared to accessions carryingFRI-wtorFRI-Colalleles. Either theFRI-Lerallele contains a mutation in cis, such as the promoter indel that defines the class, or its reduced expression is caused by the effect of trans-regulators that may occur in the early flowering accessions. In order to distinguish among these possibilities, we analyzed the allele specific expression ofFRI在混合动力车使用焦Ar的十字架abidopsis accessions differentially expressing this gene. Because expression of both alleles in a hybrid cell is controlled by the same set of regulators, the relative abundance of each allele in the hybrid should be equaled out if trans-regulators cause the differences between accessions. In contrast, if the expression differences between accessions are caused by mutations in cis, the differences in the expression of the alleles in the hybrid will be maintained [39].
We generated F1hybrids by crossing Ler-1 with Col-0 and with four accessions containingFRI-wtalleles (Bor-4, Bur-0, Sha and Knox-10; [14]). In all F1individuals analyzed the relative abundance of each allele measured in genomic DNA was close to 50%, which ensured that the assay had no preference for either of the two alleles (Figure3c). Analysis of cDNA from the same individuals revealed lower relative abundance ofFRI-Lerthan either theFRI-wtor theFRI-Colalleles (Figure3d). These differences suggest that at least part of the phenotypic differences found between accessions carrying theFRI-Lerallele and those carrying theFRI-wtallele is due to a cis regulatory mutation reducing the expression ofFRI-Ler. Interestingly, the differences in expression found among accessions classified by theirFRIallele are larger than the differences observed in allele specific expression in the hybrids (compare Figure3a and d), suggesting the existence of additional trans-regulators controlling differences in expression among alleles. Further experiments will be required to determine the precise mode of regulation of this gene.
Effect of coding polymorphisms inFRI-Ler
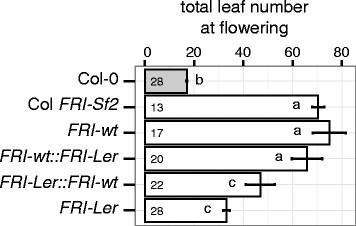
在addition to the suggested regulatory polymorphisms causing inhibition of the expression of theFRI-Lerallele, its predicted protein differs from the FRI-wt protein in the deletion of 42 amino acids from the N terminal and in one substitution located in a coiled-coil domain (Figure1d). To test whether these coding differences have an effect on the function of the FRI-Lerprotein compared to FRI-wt, we generated transgenic lines carrying constructs in which the coding region of each allele was placed downstream of the native promoter of the other allele (Figure4). Due to constraints (see Material and Methods), this experiment was performed using T1lines carryingFRI-wt(theFRI-wtallele expressed under its native promoter),FRI-wt::FRI-Ler(theFRI-Lerallele expressed under theFRI-wtpromoter) orFRI-Ler::FRI-wt(theFRI-wtallele expressed under theFRI-Lerpromoter) constructs, and individuals from two independent T3lines carrying theFRI-Lerconstruct (theFRI-Lerallele expressed under its native promoter). As expected, lines carrying the wild type promoter driving the expression of the wild type allele (FRI-wt) flowered with the same number of leaves as ColFRI-Sf2, a line in which theFRI-wtallele from the accession Sf-2 was introgressed into Col-0 [2].在addition, lines carrying constructs with theFRI-Lerpromoter flowered significantly earlier than those with alleles driven by theFRI-wtpromoter. This supports our hypothesis that cis-regulatory elements specific to theFRI-Lerpromoter are the cause of early flowering in accessions carrying this allele. Interestingly, lines carrying theFRI-Lercoding region flowered earlier than lines carrying theFRI-wtcoding region when expressed under the same promoter. Although this acceleration of flowering was not significant, it was observed both when using theFRI-Lerpromoter and theFRI-wtpromoter. This result leaves open the possibility that the loss of 42 amino acids and/or the mutation in the coiled coil domain in theFRI-Lerprotein contribute to accelerating flowering in the accessions that express it.
Flowering time of transgenic lines carrying promoter-swap constructs involving theFRI-LerandFRI-wtalleles.Plants were grown in an environmental chamber set to long day conditions and the total number of leaves (rosette + cauline) were counted on the day of the opening of their first flower. Col-0 is the untransformed control and ColFRI-Sf2is a near isogenic line containing theFRI-wtallele from the accession Sf-2 [2].Phenotypes were scored in T1individuals for lines carrying theFRI-wt,FRI-wt::FRI-LerandFRI-Ler::FRI-wtconstructs, and in individuals from two independent T3lines for lines carrying theFRI-Lerconstruct (see Material and Methods). Numbers in each bar indicate the number of individual plants analyzed per genotype. Error bars represent the standard error of the mean. Letters in each bar represent significance groups as determined by Tukey HSD test.
Analysis of life history parameters in theFRI-ColandFRI-Leraccession groups
A major question that derives from the classification ofFRI-Leras a semi-functional allele is whether it represents a change in the life history of the accessions that carry it when compared to the accessions carrying theFRI-Colallele. AlthoughFRI-Leraccessions revealed a significant higher response to vernalization than accessions with theFRI-Colallele (Figure1b), both groups only include early-flowering accessions that are not likely to differ in their general life history strategy. Nevertheless, we investigated the variation in specific life history traits between the two allelic classes, which can result in a distinct ability to adapt to local environments. To address this question, we reanalyzed fitness-related phenotypes for a panel of accessions grown at four different field locations [40].Although we observed small fitness differences in accessions carrying theFRI-ColandFRI-Leralleles at various locations, these were not significant (see Additional file4). On the other hand, accessions containing theFRI-wtallele were often significantly different in fitness traits when compared withFRI-LerandFRI-Col. Further experiments will be required to determine if differences between theFRI-LerandFRI-Colallelic classes are relevant in specific environmental conditions (e.g. under stress).
Discussion
FRI, an activator of the negative regulatorFLC, is the major determinant of natural variation in flowering time and vernalization sensitivity inArabidopsis thaliana[3].到目前为止,自然等位变异FRIhave been clustered into two groups, the functionalFRI-wtgroup and the non-functional group that included theFRI-Coland theFRI-Leralleles. Here we prove thatFRI-Ler, the most common allele found so far in summer-annual accessions, is partially functional, although it is a weak allele compared to theFRI-wtallele present in winter-annual types. We suggest that the indel found inFRI-Ler,包括启动子和一开始的一部分of the coding sequence, allows transcription of an active messenger from a downstream methionine. This resulting transcript shows lower expression and perhaps slightly reduced activity than the transcripts from bothFRI-wtandFRI-Col, but has the ability to up regulateFLC, delay flowering time and confer vernalization sensitivity. In contrast, theFRI-Colallele is unable to confer any of these phenotypes, despite being expressed at a level similar to theFRI-wtalleles present in winter-annual accessions.
AlthoughFRIhas a prominent role, not all variation inFLCexpression and flowering time observed in Arabidopsis accessions can be explained by variation at this gene. Previous studies have demonstrated that allelic variation atFLCitself provides a basis for the development of a summer-annual flowering time habit [24],[25],[41].As shown by [15], variation in flowering time may be associated withFLConly in the presence of a functionalFRIallele. Consistent with this, we observed that accessions carrying theFRI-LerorFRI-wtalleles, but not those carrying aFRI-Colallele, varied considerably inFLCexpression and flowering time (Figures1and3). Further variation ofFLClevels can be due to additional factors such as mutations in genes of the autonomous floral promotion pathway [25],[42]-[44].Moreover, allelic variation at genes directly interacting withFRI, namelySUPPRESSOR OF FRIGIDA 4 (SUF4), FRIGIDA-LIKE 1 (FRL1), FRIGIDA ESSENTIAL 1 (FES1)andFLC EXPRESSOR (FLX), could have an impact [18].For instance, it has been shown that Lercontains a non-functionalFRL1allele, which is compensated by a functionalFRIGIDA-LIKE 2 (FRL2)allele [45].The latter restoresFRI-mediated up regulation ofFLCexpression and, thus, a late flowering phenotype. In contrast, Col-0 carries a functionalFRL1allele able to interact withFRI, but an impairedFRL2allele [45].
The effect on flowering time,FLCexpression and vernalization response conferred by theFRI-Lerallele observed in the present study is not comparable to the effect of theFRI-wtallele. Although we show here that both alleles differ greatly in expression (Figure3), we detect small, non-significant but consistent differences between theFRI-Lerand theFRI-wtallele driven under the same promoter (Figure4). This suggests that the putative truncation of the first 42 aa of the N-terminal region and/or the amino acid substitution in FRI-Lercontribute to the functional difference between alleles (Figure4). In fact, previous work demonstrated that a deletion of 118 residuals from the FRI N-terminus resulted in reduced functionality. Nevertheless, plants missing that part of the protein still flowered later and revealed higherFLCexpression levels than both Col-0 and transgenic lines lacking a substantial part of the FRI C-terminus [17].Further studies used a deletion series to demonstrate that the ability of FRI to act as the scaffold protein for aFLCactivating complex depends mainly on its C-terminal part [18].These studies suggest that the truncated FRI-Lerprotein should be able to interact with its known partners to build the FRI-complex and, thus, to induceFLCexpression. Finally, the amino acid change from leucine in FRI-wt to isoleucine in FRI-Lerin the first coiled-coil domain is very conservative, but might impair the functionality of the protein (Figure1d). However, the small differences observed between theFRI-LerandFRI-wtalleles expressed under the same promoter suggest that if real, the effect of these mutations on flowering time is minor compared to the effect of their differences in expression.
Despite the functional difference between the two alleles, accessions of theFRI-ColandFRI-Lergroup are all rapid cycling and, thus, do not display a difference in their general life history strategy. Furthermore, differences in fitness traits between both allelic groups could not be detected in the present study. A more comprehensive analysis of larger data sets collected in multiple environments might be required to detect a distinct effect of theFRI-Lerallele in nature. In fact, [46已经证明,这取决于seasonal timing of specific environmental signals, Arabidopsis accessions in nature are capable of both life history strategies. For instance, Col-0 growing during autumn in the field at a specific location in Germany (Halle) displayed a winter-annual habit and flowered almost at the same time as the introgression line ColFRI-Sf2that carries a functionalFRI-wtallele [46].在contrast, ColFRI-Sf2exhibited an unexpected rapid-cycling phenotype when grown in summer at one location in England (Norwich). Nevertheless, Lerresembled Col-0 with regard to flowering time at all locations tested [46].Furthermore, we cannot exclude that theFRI-Lerallele has a role in the modulation of adaptive parameters other than flowering time and vernalization response. For example, pleiotropic effects ofFRIhave been reported for traits such as water use efficiency and leaf senescence, where the latter is closely linked to plant reproduction [47],[48].
Conclusions
在the present study we disprove the common assumption that the widespreadFRIallele found in the Arabidopsis Leraccession is not functional. We demonstrate that theFRI-Lerallele increases flowering time andFLCexpression and induces vernalization responses to levels that are in between the ones observed for the true nullFRI-Colallele and the fully functionalFRI-wtallele.
These intermediate phenotypes observed in plants carryingFRI-Lercould be explained by the presence of a close to full-length protein never before associated with this allele. We show that the differences in functionality between theFRI-Lerand theFRI-wtalleles are largely due to expression polymorphisms, although variation in the protein sequence may also play a role.
Using the limited data available from plants grown under natural conditions, we cannot conclude that theFRI-Lerallele confers differences in fitness or life history strategies when compared to theFRI-Colallele. On the other hand, phenotypic differences between accessions carrying these alleles are clear and could be advantageous under specific natural environments not explored so far.
在summary, we demonstrate the existence of an allelic series inFRI. Besides completely functional and non-functional alleles, we have found a widespread allelic class with intermediate functionality. The use of this novel classification will increase the accuracy of adaptive studies in Arabidopsis.
Methods
Analysis of published datasets
Leaf number from Recombinant Inbred Lines (RILs) in the Lerx Col population [31]分析了分组行寡糖r genotypes at the closest molecular markers toFRI(m506, chromosome 4 at 0.0 cM) andFLC(g4560, chromosome 5 at 17.3 cM). Phenotypic and genotypic data for these individuals was kindly provided by Johan W. van Ooijen and Caroline Dean.
从126年春化反应Arabidopsis accessions were obtained from published data [22] and analyzed by subtracting days to flowering with vernalization from days to flowering without vernalization (plants grown in controlled environment rooms with 16 hour light). In this dataset,FRIalleles are classified asFRI-Ler,FRI-Col, non-functional alleles or novel alleles. The rest of the alleles were assumed to be functional (FRI-wt) as they do not contain the Leror Col-0 indels and the accessions that carry them are late flowering. For our analysis, we considered only those accessions containing alleles classified asFRI-wt,FRI-LerorFRI-Col. Accessions for which theFLCallele was classified as non-functional or novel were removed.
Expression data forFRIandFLCwas obtained from available repositories holding northern hybridization quantification for 137 Arabidopsis accessions (greenhouse grown, 16 h light [38]). Accessions in this dataset were classified according to theirFRIalleles by combining genotypes from the following sources: [14],[21],[22],[27],[37].The assignment ofFRIalleles for each accession can be found in Additional file5.
Fitness data was obtained from Arabidopsis accessions grown in four different field locations [40].These accessions were classified according to theirFRIalleles as above.
Sequencing ofFRIin Col and Ler
Genomic DNA of Col-0 and Ler-1 was extracted from young leaves using a Plant DNeasy Mini Kit (Qiagen, Chatsworth, CA, USA). A region of approximately 3250 bp including the completeFRIgene (AT4G00650) and an upstream and downstream region was amplified in overlapping fragments of 600–700 bp using Phusion High-Fidelity DNA Polymerase (New England Biolabs). As a reference, the same region was sequenced for the accession Bil-7 carrying a fully functionalFRI-wtallele [14].Pooled PCR products from four independent reactions were purified using QIAquick PCR purification kit (Qiagen, Chatsworth, CA, USA) and sequenced via Sanger sequencing at the Max Planck Genome Centre Cologne. The individual sequences were assembled and aligned against the sequence of Bil-7 using SeqMan Pro (DNAstar, Madison, WI, USA).
Cloning and phenotyping of transgenics
在order to clone theFRI-Ler, FRI-wtandFRI-Colalleles, we designed primers flanking positions -1372 to +2691 relative to theFRIstart codon annotated in TAIR10, which include the complete gene plus an upstream region of 1061 bp and a downstream region of 379 bp. This region was amplified by PCR from DNA of the accessions Ler-1, Bil-7 and Col-0 using Phusion High-Fidelity DNA Polymerase (New England Biolabs). ForFRI-LerandFRI-Col, PCR fragments were introduced into the binary vector pCAMBIA2300 making use of EcoRI/BamHI restriction sites. ForFRI-wt, the PCR fragment was introduced into binary vector pBinGlyRed1 (kindly provided by Ed Cahoon, University of Nebraska) making use of the same EcoRI/BamHI restriction sites.
Promoter-swap constructs were generated using the MultiSite Gateway®Pro 2.0 system (Life Technologies) and genomic DNA from the accessions Ler-1 (FRI-Ler)和Bil-7 (FRI-wt). TheFRI-Lerpromoter region was cloned starting from position -1372, relative to the annotatedFRIstart codon in TAIR10, and ending at position +126, which is right before the first ATG downstream of the indel described inFRI-Ler. ForFRI-wt,the promoter region cloned ranged between positions -1372 and -1. Both promoter sequences were introduced into pDONRTM 221 P1-P5r by BP reaction. Similarly, the coding sequences for both alleles together with 379 bp of its downstream sequence (position +1 to position +2691 bp forFRI-wtand position +127 to position +2691 forFRI-Ler) were cloned and introduced into pDONRTM 221 P5-P2. Finally,FRI-wt::FRI-LerandFRI-Ler::FRI-wtconstructs were generated from LR reactions using the binary vector pFAST.
All final constructs were transformed intoE. colistrain Top10 (One Shot® TOP10 chemically competentE. coli, Invitrogen). Inserts from positive colonies were sequenced and verified by comparing them to the PCR template sequence or to the expected in silico constructs. Subsequently, all constructs were transformed intoAgrobacterium tumefaciensstrain GV3101 and used for transformation of Col-0 plants by the floral dip method [49].
Transgenic plants carryingFRI-LerorFRI-Colconstructs (in pCAMBIA) were selected on MS agar plates containing 50 mg/l kanamycin. Independent T1plants carrying a single insertion were identified and homozygous T3plants were used for subsequent analyses. Flowering time in T3lines carryingFRI-ColorFRI-Lerwas measured in the greenhouse under long day conditions (16 h day length) with and without vernalization treatment for four weeks at 4°C.
T1plants carrying the promoter swap betweenFRI-LerandFRI-wt(in pFAST) and those carrying the Bil-7FRI-wtalleles (in pBinGlyRed1) were selected by their red fluorescence under a fluorescence stereomicroscope (Leica MZ16F, Leica Microsystems, Germany) using green light of wavelength about 580 nm. Flowering time was recorded in T1lines carrying theFRI-wtallele and the promoter swap linesFRI-wt:FRI-LerandFRI-Ler::FRI-wtunder long day condition in an environmental chamber. This experiment included plants from two independent T3lines carrying theFRI-Ler::FRI-Lerallele (lines 14–1 and 16–4 in Figure2). Using these T3lines allowed us to sow all the plants in the experiment directly on soil, asFRI-LerT1行没有红色荧光标记和have required selection on kanamycin plates.
在all experiments, flowering time was quantified as total leaf number (rosette + cauline) on the day the first flower opened.
Expression analysis using quantitative RT-PCR
For expression analysis of transgenics, seedlings (leaves plus shoot) grown in an environmental chamber at long day conditions (16 h light) were harvested 10 days after sowing. Material of 8 to 10 seedlings was pooled for each of three biological replicates. RNA was extracted using Trizol (Ambion®TRIzol®RNA Isolation Reagent, Life Technologies) and transcribed into cDNA using Super Script®II Reverse Transcriptase (Invitrogen). Quantitative RT-PCR was performed on a CFX384 Touch™ Real-Time PCR Detection System using SYBR Green dye (iQ™ SYBR®Green Supermix, Biorad) using the following gene-specific primers:FLC-fwd: 5′-CCGAACTCATGTTGAAGCTTGTTGAG-3′,FLC-rev: 5′- CGGAGATTTGTCCAGCAGGTG-3′,FRI-fwd: 5′- TGCCTGATCGTGGTAAAGGGAAG-3′ andFRI-rev: 5′- AGCACCGGCAATCTCATTCGAAC-3′. Expression values were determined using the standard curve method and normalized to the expression ofPP2A(PP2A-fwd: 5′-TAACGTGGCCAAAATGATGC-3′,PP2A-rev: 5′- GTTCTCCACAACCGCTTGGT-3′). Normalized expression was averaged for three biological replicates each analyzed in two or three technical replicates.
Allele-specific expression analysis
Leaves from F1hybrids involving the Ler-1 parent were collected at bolting from plants grown in 12 h days in a controlled environmental chamber. Material from F1hybrids involving the Col-0 parent were collected from ten-day old seedlings grown in long days (16 h light) in a controlled environmental chamber. Here, material of 8 to 10 seedlings was pooled per biological replicate. RNA was extracted from 20 mg of frozen material using RNeasy Plant kit (Qiagen) combined with on-column DNase digestion using RNase-Free DNase Set (Qiagen). Subsequently, cDNA was synthesized using SuperScript®III Reverse Transcriptase kit (Invitrogen) in the presence of RNase inhibitor RNasin (Promega) following specifications from the manufacturer. Genomic DNA was extracted from the same samples using DNease Plant kit (Qiagen).
PCR distinguishingFRI-LerversusFRI-wtalleles targeted a variant from C (in Bor-4, Bur-0, Sha and Knox-10) to A (in Ler-1) at chromosome 4, position 269257 (TAIR10). The fragment containing this SNP was amplified using primers 5′Biotin-TCAGTTGCAGTGGAAACATTCA-3′ and 5′-GCGTTTTCGATTGACTCGATGT-3′, and pyrosequencing was performed using primer 5′-TGACTCGATGTGCTTCT-3′. To distinguish theFRI-LerandFRI-Colalleles in the Lerx Col F1hybrid we targeted a variant from A in Col-0 to G in Ler-1 at chromosome 4, position 269469 (TAIR10). The fragment containing this SNP was amplified using primers 5′Biotin-ATTGTACCGGAGACGTCGAATAA-3′ and 5′-GGCCAATTTCAAAGCTGAAG-3′, and pyrosequencing was performed with primer 5′-CTTTGCTACACATCAACTC-3′. 0.5 μL of cDNA was used for PCR, which was conducted in a solution (2.5 units of Taq polymerase, 2.5 mM MgCl2, 200 μM dNTPs, buffer B, 0.2 mM of each dNTP; Bio-Budget) containing 20 pmol of each primer, in the total volume of 25 μL. The PCR products were obtained with 50 cycles (93°C, 45 sec; 60°C, 45 sec; 72°C, 1 min).
Allele-specific mRNA abundances were measured using PyrosequencerAB (Biotage AB, now Qiagen) following manufacturer's instructions for sample preparation and pyrosequencing reactions. Vacuum sample preparation was performed using 15 μL of PCR product mixed with 5 μL of Streptavidin Sepharose beads (GE Healthcare), 40 μL of PyroMark Binding Buffer (Qiagen) and 20 μL of LiChroSolv water (Merck). Pyrosequencing was performed in a PyroMark Q96 Plate Low (Qiagen) in each well containing 1 μL of sequencing primer (10 μM) and 40 μL of Pyromark Annealing Buffer (Qiagen) using PyroMark Gold Q96 Reagents (Qiagen). Allele-specific expression for each SNP was estimated using the pyrosequencing software (PSQ 96MA 2.1.1, Biotage AB) based on the peak height for each allele at the SNP. A peak correction factor of 0.86 was used for incorporation of dATP αS, as recommended by the manufacturer.
Availability of supporting data
The coding DNA sequence and translated protein sequence of theFRI-Lerallele supporting the results of this article are available through NCBI's GenBank under accession number KJ545576 (http://www.ncbi.nlm.nih.gov/genbank).
Additional files
References
Hoffmann MH: Biogeography of Arabidopsis thaliana (L.) Heynh. (Brassicaceae). J Biogeogr. 2002, 29 (1): 125-134. 10.1046/j.1365-2699.2002.00647.x.
Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C: Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science. 2000, 290 (5490): 344-347. 10.1126/science.290.5490.344.
Michaels SD, Amasino RM: FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999, 11 (5): 949-956. 10.1105/tpc.11.5.949.
Deng W, Ying H, Helliwell CA, Taylor JM, Peacock WJ, Dennis ES: FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc Natl Acad Sci U S A. 2011, 108 (16): 6680-6685. 10.1073/pnas.1103175108.
Helliwell CA, Wood CC, Robertson M, James Peacock W, Dennis ES: The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 2006, 46 (2): 183-192. 10.1111/j.1365-313X.2006.02686.x.
Hepworth SR, Valverde F, Ravenscroft D, Mouradov A, Coupland G: Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 2002, 21 (16): 4327-4337. 10.1093/emboj/cdf432.
Andres F, Coupland G: The genetic basis of flowering responses to seasonal cues. Nat Rev Genet. 2012, 13 (9): 627-639. 10.1038/nrg3291.
Crevillen P, Dean C: Regulation of the floral repressor gene FLC: the complexity of transcription in a chromatin context. Curr Opin Plant Biol. 2011, 14 (1): 38-44. 10.1016/j.pbi.2010.08.015.
Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C: Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004, 427 (6970): 164-167. 10.1038/nature02269.
Heo JB, Sung S: Encoding memory of winter by noncoding RNAs. Epigenetics. 2011, 6 (5): 544-547. 10.4161/epi.6.5.15235.
Swiezewski S, Liu F, Magusin A, Dean C: Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature. 2009, 462 (7274): 799-802. 10.1038/nature08618.
Angel A, Song J, Dean C, Howard M: A Polycomb-based switch underlying quantitative epigenetic memory. Nature. 2011, 476 (7358): 105-108. 10.1038/nature10241.
Coustham V, Li P, Strange A, Lister C, Song J, Dean C: Quantitative modulation of polycomb silencing underlies natural variation in vernalization. Science. 2012, 337 (6094): 584-587. 10.1126/science.1221881.
Shindo C, Aranzana MJ, Lister C, Baxter C, Nicholls C, Nordborg M, Dean C: Role of FRIGIDA and FLOWERING LOCUS C in determining variation in flowering time of Arabidopsis. Plant Physiol. 2005, 138 (2): 1163-1173. 10.1104/pp.105.061309.
Caicedo AL, Stinchcombe JR, Olsen KM, Schmitt J, Purugganan MD: Epistatic interaction between Arabidopsis FRI and FLC flowering time genes generates a latitudinal cline in a life history trait. Proc Natl Acad Sci U S A. 2004, 101 (44): 15670-15675. 10.1073/pnas.0406232101.
Sheldon CC, Conn AB, Dennis ES, Peacock WJ: Different regulatory regions are required for the vernalization-induced repression of FLOWERING LOCUS C and for the epigenetic maintenance of repression. Plant Cell. 2002, 14 (10): 2527-2537. 10.1105/tpc.004564.
Risk JM, Laurie RE, Macknight RC, Day CL: FRIGIDA and related proteins have a conserved central domain and family specific N- and C- terminal regions that are functionally important. Plant Mol Biol. 2010, 73 (4-5): 493-505. 10.1007/s11103-010-9635-2.
崔K金J,黄HJ,金正日年代,公园C, Kim SY勒e I: The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors. Plant Cell. 2011, 23 (1): 289-303. 10.1105/tpc.110.075911.
Jiang D, Gu X, He Y: Establishment of the winter-annual growth habit via FRIGIDA-mediated histone methylation at FLOWERING LOCUS C in Arabidopsis. Plant Cell. 2009, 21 (6): 1733-1746. 10.1105/tpc.109.067967.
Geraldo N, Baurle I, Kidou S, Hu X, Dean C: FRIGIDA delays flowering in Arabidopsis via a cotranscriptional mechanism involving direct interaction with the nuclear cap-binding complex. Plant Physiol. 2009, 150 (3): 1611-1618. 10.1104/pp.109.137448.
Hagenblad J, Tang C, Molitor J, Werner J, Zhao K, Zheng H, Marjoram P, Weigel D, Nordborg M: Haplotype structure and phenotypic associations in the chromosomal regions surrounding two Arabidopsis thaliana flowering time loci. Genetics. 2004, 168 (3): 1627-1638. 10.1534/genetics.104.029470.
Lempe J, Balasubramanian S, Sureshkumar S, Singh A, Schmid M, Weigel D: Diversity of flowering responses in wild Arabidopsis thaliana strains. PLoS Genet. 2005, 1 (1): 109-118. 10.1371/journal.pgen.0010006.
Le Corre V, Roux F, Reboud X: DNA polymorphism at the FRIGIDA gene in Arabidopsis thaliana: extensive nonsynonymous variation is consistent with local selection for flowering time. Mol Biol Evol. 2002, 19 (8): 1261-1271. 10.1093/oxfordjournals.molbev.a004187.
Mendez-Vigo B, Pico FX, Ramiro M, Martinez-Zapater JM, Alonso-Blanco C: Altitudinal and climatic adaptation is mediated by flowering traits and FRI, FLC, and PHYC genes in Arabidopsis. Plant Physiol. 2011, 157 (4): 1942-1955. 10.1104/pp.111.183426.
Gazzani S, Gendall AR, Lister C, Dean C: Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol. 2003, 132 (2): 1107-1114. 10.1104/pp.103.021212.
Stinchcombe JR, Weinig C, Ungerer M, Olsen KM, Mays C, Halldorsdottir SS, Purugganan MD, Schmitt J: A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proc Natl Acad Sci U S A. 2004, 101 (13): 4712-4717. 10.1073/pnas.0306401101.
Toomajian C, Hu TT, Aranzana MJ, Lister C, Tang C, Zheng H, Zhao K, Calabrese P, Dean C, Nordborg M: A nonparametric test reveals selection for rapid flowering in the Arabidopsis genome. PLoS Biol. 2006, 4 (5): e137-10.1371/journal.pbio.0040137.
Werner JD, Borevitz JO, Uhlenhaut NH, Ecker JR, Chory J, Weigel D: FRIGIDA-independent variation in flowering time of natural Arabidopsis thaliana accessions. Genetics. 2005, 170 (3): 1197-1207. 10.1534/genetics.104.036533.
El-Lithy我,Bentsink L, Hanhart CJ, ruy GJ, Rovito D, Broekhof JL, van der Poel HJ, van Eijk MJ, Vreugdenhil D, Koornneef M: New Arabidopsis recombinant inbred line populations genotyped using SNPWave and their use for mapping flowering-time quantitative trait loci. Genetics. 2006, 172 (3): 1867-1876. 10.1534/genetics.105.050617.
Tisne S, Schmalenbach I, Reymond M, Dauzat M, Pervent M, Vile D, Granier C: Keep on growing under drought: genetic and developmental bases of the response of rosette area using a recombinant inbred line population. Plant Cell Environ. 2010, 33 (11): 1875-1887. 10.1111/j.1365-3040.2010.02191.x.
Jansen RC, Van Ooijen JW, Stam P, Lister C, Dean C: Genotype-by-environment interaction in genetic mapping of multiple quantitative trait loci. Theor Appl Genet. 1995, 91 (1): 33-37. 10.1007/BF00220855.
Koornneef M, Blankestijn-de Vries H, Hanhart C, Soppe W, Peeters T: The phenotype of some late-flowering mutants is enhanced by a locus on chromosome 5 that is not effective in the Landsberg erecta wild-type. Plant J. 1994, 6 (6): 911-919. 10.1046/j.1365-313X.1994.6060911.x.
Liu J, He Y, Amasino R, Chen X: siRNAs targeting an intronic transposon in the regulation of natural flowering behavior in Arabidopsis. Genes Dev. 2004, 18 (23): 2873-2878. 10.1101/gad.1217304.
Lee I, Amasino RM: Effect of vernalization, photoperiod, and light quality on the flowering phenotype of arabidopsis plants containing the FRIGIDA gene. Plant Physiol. 1995, 108 (1): 157-162.
Lee I, Bleecker A, Amasino R: Analysis of naturally occurring late flowering in Arabidopsis thaliana. Mol Gen Genet. 1993, 237 (1-2): 171-176.
Gan X, Stegle O, Behr J, Steffen JG, Drewe P, Hildebrand KL, Lyngsoe R, Schultheiss SJ, Osborne EJ, Sreedharan VT, Kahles A, Bohnert R, Jean G, Derwent P, Kersey P, Belfield EJ, Harberd NP, Kemen E, Toomajian C, Kover PX, Clark RM, Rätsch G, Mott R: Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature. 2011, 477 (7365): 419-423. 10.1038/nature10414.
Korves TM,施密德KJ, Caicedo, Mays C, Stinchcombe JR, Purugganan MD, Schmitt J: Fitness effects associated with the major flowering time gene FRIGIDA in Arabidopsis thaliana in the field. Am Nat. 2007, 169 (5): E141-E157. 10.1086/513111.
Atwell S, Huang YS, Vilhjalmsson BJ, Willems G, Horton M, Li Y, Meng D, Platt A, Tarone AM, Hu TT, Jiang R, Muliyati NW, Zhang X, Amer MA, Baxter I, Brachi B, Chory J, Dean C, Debieu M, de Meaux J, Ecker JR, Faure N, Kniskern JM, Jones JDG, Michael T, Nemri A, Roux F, Salt DE, Tang C, Todesco M, et al: Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010, 465 (7298): 627-631. 10.1038/nature08800.
Wittkopp PJ, Haerum BK, Clark AG: Evolutionary changes in cis and trans gene regulation. Nature. 2004, 430 (6995): 85-88. 10.1038/nature02698.
Fournier-Level A, Korte A, Cooper MD, Nordborg M, Schmitt J, Wilczek AM: A map of local adaptation in Arabidopsis thaliana. Science. 2011, 334 (6052): 86-89. 10.1126/science.1209271.
Ehrenreich IM, Hanzawa Y, Chou L, Roe JL, Kover PX, Purugganan MD: Candidate gene association mapping of Arabidopsis flowering time. Genetics. 2009, 183 (1): 325-335. 10.1534/genetics.109.105189.
Lee I, Aukerman MJ, Gore SL, Lohman KN, Michaels SD, Weaver LM, John MC, Feldmann KA, Amasino RM: Isolation of LUMINIDEPENDENS: a gene involved in the control of flowering time in Arabidopsis. Plant Cell. 1994, 6 (1): 75-83. 10.1105/tpc.6.1.75.
Macknight R, Bancroft I, Page T, Lister C, Schmidt R, Love K, Westphal L, Murphy G, Sherson S, Cobbett C, Dean C: FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell. 1997, 89 (5): 737-745. 10.1016/S0092-8674(00)80256-1.
Simpson GG, Dijkwel PP, Quesada V, Henderson I, Dean C: FY is an RNA 3′ end-processing factor that interacts with FCA to control the Arabidopsis floral transition. Cell. 2003, 113 (6): 777-787. 10.1016/S0092-8674(03)00425-2.
Schlappi MR: FRIGIDA LIKE 2 is a functional allele in Landsberg erecta and compensates for a nonsense allele of FRIGIDA LIKE 1. Plant Physiol. 2006, 142 (4): 1728-1738. 10.1104/pp.106.085571.
Wilczek AM, Roe JL, Knapp MC, Cooper MD, Lopez-Gallego C, Martin LJ, Muir CD, Sim S, Walker A, Anderson J, Egan JF, Moyers BT, Petipas R, Giakountis A, Charbit E, Coupland G, Welch SM, Schmitt J: Effects of genetic perturbation on seasonal life history plasticity. Science. 2009, 323 (5916): 930-934. 10.1126/science.1165826.
Wingler A, Purdy SJ, Edwards SA, Chardon F, Masclaux-Daubresse C: QTL analysis for sugar-regulated leaf senescence supports flowering-dependent and -independent senescence pathways. New Phytol. 2010, 185 (2): 420-433. 10.1111/j.1469-8137.2009.03072.x.
Lovell JT, Juenger TE, Michaels SD, Lasky JR, Platt A, Richards JH, Yu X, Easlon HM, Sen S, McKay JK: Pleiotropy of FRIGIDA enhances the potential for multivariate adaptation. Proc Biol Sci/ Royal Soc. 2013, 280 (1763): 20131043-10.1098/rspb.2013.1043.
Clough SJ, Bent AF: Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16 (6): 735-743. 10.1046/j.1365-313x.1998.00343.x.
Acknowledgements
We thank Caroline Dean and Johan W. Ooijen for kindly providing the phenotypic and genotypic data of the Lerx Col RIL population. Furthermore, we thank Ute Tartler and Regina Gentges for plant handling. We are grateful to Maarten Koornneef, Niels Müller and Jinyong Hu for discussions during the course of the work and for comments on the manuscript. IS acknowledges support from the German Research Foundation (DFG project number SCHM2793/1-1) and the Max Planck Society. LZ was funded by an International Max Planck Research School PhD fellowship. MR received funding from the German Research Foundation under the German-Israeli Project Cooperation program (DFG DIP project number FE552/12-1).
Author information
Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
IS cloned the naturalFRIalleles and wrote the manuscript. LZ generated the promoter-swap lines, selected and phenotyped all transgenic lines and performed the expression analyses. MR conducted allele-specific expression analyses. JMJG supervised the work, analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12870_2014_218_MOESM1_ESM.zip
Additional file 1:Response to vernalization quantified as the decrease in the total number of leaves at flowering for Arabidopsis accessions grouped by theirFRIallele, as described in [[22]].(子P 131 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visithttps://creativecommons.org/licenses/by/4.0/.
The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schmalenbach, I., Zhang, L., Ryngajllo, M.et al.Functional analysis of the Landsbergerectaallele ofFRIGIDA.BMC Plant Biol14,218 (2014). https://doi.org/10.1186/s12870-014-0218-2
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/s12870-014-0218-2
Keywords
- Arabidopsis thaliana
- Flowering time
- FRIGIDA
- Vernalization
- Natural variation
- Allelic series