- Research article
- Open Access
- Published:
表皮蜡抑制剂轨迹Iw2in wild diploid wheatAegilops tauschii: phenotypic survey, genetic analysis, and implications for the evolution of common wheat
BMC Plant Biologyvolume14, Article number:246(2014)
Abstract
Background
Cuticular wax production on plant surfaces confers a glaucous appearance and plays important roles in plant stress tolerance. Most common wheat cultivars, which are hexaploid, and most tetraploid wheat cultivars are glaucous; in contrast, a wild wheat progenitor,Aegilops tauschii, can be glaucous or non-glaucous. A dominant non-glaucous allele,Iw2, resides on the short arm of chromosome 2D, which was inherited fromAe。tauschiithrough polyploidization.Iw2is one of the major causal genes related to variation in glaucousness among hexaploid wheat. Detailed genetic and phylogeographic knowledge of theIw2locus inAe。tauschiimay provide important information and lead to a better understanding of the evolution of common wheat.
Results
GlaucousAe。tauschiiaccessions were collected from a broad area ranging from Armenia to the southwestern coastal part of the Caspian Sea. Linkage analyses with five mapping populations showed that the glaucous versus non-glaucous difference was mainly controlled by theIw2locus inAe。tauschii。Comparative genomic analysis of barley andAe。tauschiiwas then used to develop molecular markers tightly linked withAe。tauschii Iw2。Chromosomal synteny around the orthologousIw2regions indicated that some chromosomal rearrangement had occurred during the genetic divergence leading toAe。tauschii, barley, andBrachypodium。Genetic associations between specificIw2-linked markers and respective glaucous phenotypes inAe。tauschiiindicated that at least two non-glaucous accessions might carry other glaucousness-determining loci outside of theIw2locus.
Conclusion
Allelic differences at theIw2locus were the main contributors to the phenotypic difference between the glaucous and non-glaucous accessions ofAe。tauschii。Our results supported the previous assumption that the D-genome donor of common wheat could have been anyAe。tauschiivariant that carried the recessiveiw2allele.
Background
Cuticular wax production on aerial surfaces of plants has important roles in various physiological functions and developmental events; the wax prevents non-stomatal water loss, inhibits organ fusion during development, protects from UV radiation damage, and imposes a physical barrier against pathogenic infection [1]-[4]。The trait, the coating of leaf and stem surfaces with a waxy whitish substance, is called glaucousness. In common wheat (Triticum aestivumL., 2n = 6x = 42, genome constitution BBAADD), dominant allelesW1andW2, control the wax production and have been assigned to chromosomes 2B and 2D, respectively [5],[6]。Additionally, dominant homoeoalleles for non-glaucousness,Iw1andIw2, have also been mapped to the short arms of chromosomes 2B and 2D, respectively [6]-[9]。Wheat plants with either thew1, w2,Iw1orIw2allele show the non-glaucous phenotype, indicating thatW1andW2are functionally redundant for the glaucous phenotype and that a singleIwdominant allele is sufficient to inhibit the glaucous phenotype even in the presence of aW1orW2allele [3],[6]。Wax composition in wheat plants with oneIwdominant allele is biochemically different from that in glaucous plants of any genotype; ß-diketones are completely absent from extracts of cuticular wax fromIwplants, while aldehydes and primary alcohols are very abundant in these extracts [3],[10]。A fine map around theIw1region on 2BS was constructed using an F2population of tetraploid wheat (Triticum turgidumL., 2n = 4x =28, BBAA), and three markers tightly linked toIw1were developed [10],[11]。A high-resolution map ofIw2on 2DS has been developed in hexaploid wheat, and two markers tightly linked toIw2were also developed [11]。Comparative mapping ofIw1andIw2shows that the two loci are homoeologous to each other and orthologous to the same chromosomal region ofBrachypodium distachyon(L.) P. Beauv. [11]。Recently, a third wax-inhibitor locusIw3was identified on chromosome 1BS from wild emmer wheat [12], and a fine map of theIw3locus is available [13]。Iw2is located on 2DS inAegilops tauschiiCoss. (2n = 2x = 14, DD), which is diploid and the progenitor of the D-genome of common wheat [14], but to our knowledge, a high-resolution genetic map of theIw2region inAe。tauschiihas not been constructed.
Common wheat is an allohexaploid species derived from interspecific hybridization between tetraploid wheat with a BBAA genome andAe。tauschii。Most cultivated varieties of tetraploid wheat are glaucous, even though non-glaucous types are frequently found among wild tetraploid accessions [6],[15]; this variation indicates that the glaucous phenotype might have been a target of artificial selection during the domestication of tetraploid wheat. Glaucous accessions ofAe。tauschiiare found in the area ranging from Transcaucasia to the southern coastal region of the Caspian Sea [5],[16]。Almost all varieties of common wheat carryW1andW2and lackIw1andIw2; therefore, the D-genome donor of common wheat is assumed to have had the recessiveiw2allele [5]。GlaucousAe。tauschiiaccessions have theW2andiw2alleles. Non-glaucous accessions ofAe。tauschiithat have theW2andIw2alleles have been recovered from a wide distribution range in central Eurasia [5]。Moreover, discovery of a non-glaucousAe。tauschiiaccession with thew2recessive allele has not yet been reported.
Therefore, analysis of theIw2locus may provide important information that improves our understanding of the evolution of common wheat. Population structure analyses ofAe。tauschiiindicate that the whole speciesAe。tauschiican be divided into three major genealogical lineages,tauschiilineage 1 (TauL1), TauL2, and TauL3, and that genetically genomes of TauL2 accessions are most closely related to the D genome of common wheat [17]-[19]。Recently, a whole-genome shotgun strategy was used to generate a draft genome sequence ofAe。tauschiithat has been published; this draft anchors 1.72 Gb of the 4.36 Gb genome to chromosomes [20]。A physical map of theAe。tauschiigenome that covers 4 Gb is also available [21]。The objectives of this study were (1) to examine the natural variation in glaucousness among a species-wide set ofAe。tauschiiaccessions, (2) to use F2populations ofAe。tauschiiaccessions and synthetic hexaploid wheat lines to fine-mapIw2locus on 2DS, (3) to develop molecular markers that are closely linked toIw2based on chromosomal synteny between barley and wheat chromosomes, and (4) to provide novel insights into the evolutionary relationship between theAe。tauschiigenome and the D genome of common wheat on the basis of the detailed genetic and phylogeographic knowledge of theIw2chromosomal region.
Methods
Plant materials and phenotype evaluation
In all, 210Ae。tauschiiaccessions were used in this study [22]。Their passport data, including geographical coordinates, have been provided in previous reports [23],[24]。Previously, 206 of theAe。tasuchii登记入册,分为三个血统,TauL1, TauL2, and TauL3, based on DArT marker genotyping analysis [19]。Of the 210 accessions, 12 were previously identified as subspeciesstrangulatabased on the sensu-strico criteria [25],[26]。Seeds from twoAe。tauschiihybrid F2populations (n = 116 from each population) were sown in November 2011; one F2population resulted from a cross between KU-2154 (non-glaucous) and KU-2126 (glaucous), the other from a KU-2003 (non-glaucous) by KU-2124 (glaucous) cross. In the 2012–2013 season, 169 additional F2individuals of the KU-2154/KU-2126 population were grown to increase the size of the mapping population.
Previously, 82 synthetic hexaploid wheat lines were produced from crosses between a tetraploid wheat (T. turgidumsubspeciesdurum(Desf.) Husn.) cultivar Langdon (Ldn) and 69Ae。tauschiiaccessions [26],[27]。These synthetic hexaploid wheat lines were used for crossing and phenotypic studies conducted in a glasshouse at Kobe University. Ldn shows the glaucous phenotype and is homozygous for theiw1allele [10]。Each synthetic hexaploid thus contained the A and B genomes from Ldn and one of many diverse D genomes originating from theAe。tauschiipollen parents. In the present study, four F3plants derived from one F2plant of each synthetic hexaploid were grown individually during the 2007–2008 season in pots that were arranged randomly in the glasshouse; these 276 F3plants were used for crossing and phenotypic observation. The following three pairs of synthetic hexaploids were used to generate three F2mapping populations: Ldn/PI476874 (non-glaucous) and Ldn/KU-2069 (glaucous), Ldn/IG126387 (non-glaucous) and Ldn/KU-2159 (glaucous), and Ldn/KU-2124 (glaucous) and Ldn/IG47259 (non-glaucous). The first population (Ldn/PI476874//Ldn/KU-2069) comprised 106 F2individuals grown in the glasshouse during the 2009–2010 season. Seeds from the other two populations were sown in November 2011, with the numbers of individuals in each being 100 (Ldn/KU-2159//Ldn/IG126387) and 82 (Ldn/KU-2124//Ldn/IG47259).
For analysis of the D genome of common wheat, 17 landraces collected in Iran were supplied from the National BioResource Project (NBRP) KOMUGI (http://www.shigen.nig.ac.jp/wheat/komugi).These Iranian landraces—KU-3097, KU-3098, KU-3121, KU-3126, KU-3136, KU-3162, KU-3184, KU-3189, KU-3202, KU-3232, KU-3236, KU-3274, KU-3289, KU-10393, KU-10439, KU-10480, and KU-10510—each showed the glaucous phenotype.
Glaucousness was evaluated based on the presence or absence of wax production on the surface of peduncles and spikes in bothAe。tauschiiand synthetics. Wax production was clearly visible and whitish.
Genotyping and construction of linkage maps
To amplify PCR fragments containing molecular markers, some of which were simple sequence repeats (SSRs), total DNA was extracted from leaves of the parental strains and F2individuals. For SSR genotyping, 40 cycles of PCR were performed using 2x Quick Taq HS DyeMix (TOYOBO, Osaka, Japan) and the following conditions: 10 s at 94°C, 30 s at the appropriate annealing temperature (72, 73, or 75°C), and 30 s at 68°C. The last step was a 1-min incubation at 68°C. Information on SSR markers and the respective annealing temperatures was obtained from the NBRP KOMUGI web site (http://www.shigen.nig.ac.jp/wheat/komugi/strains/aboutNbrpMarker.jsp) and the GrainGenes web site (http://wheat.pw.usda.gov/GG2/maps.shtml).PCR products were resolved in 2% agarose or 13% nondenaturing polyacrylamide gels and visualized under UV light after staining with ethidium bromide. The MAPMAKER/EXP version 3.0b package was used for genetic mapping [28]。The threshold for log-likelihood scores was set at 3.0, and genetic distances were calculated with the Kosambi function [29]。
Each polymorphism at thePpd-D1locus on 2DS was detected with allele-specific primers and methodology described by Beales et al. [30]。A common forward primer, Ppd-D1_F (5′-ACGCCTCCCACTACACTG-3′), and two reverse primers, Ppd-D1_R1 (5′-GTTGGTTCAAACAGAGAGC-3′) and Ppd-D1_R2 (5′-CACTGGTGGTAGCTGAGATT-3′), were used for this PCR analysis. PCR products amplified with Ppd-D1_F and Ppd-D1_R2 detected a 2,089-bp deletion in the 5′ upstream region ofPpd-D1that is indicative of the photoperiod-insensitivePpd-D1aallele [30]。EST-derived sequence-tagged site (STS) markers on 2DS, TE6, and WE6 were also used for genotyping; two STS markers for each locus, and these markers were previously developed along with theIw2-linked markers [7]。The amplified PCR products were separated via electrophoresis through a 2% agarose or 13% nondenaturing polyacrylamide gel and then stained with ethidium bromide.
Development of additional markers linked to Iw2
In our previous studies, we conducted deep-sequencing analyses of the leaf and spike transcriptomes of twoAe。tauschiiaccessions that represented two major lineages, and discovered more than 16,000 high-confidence single nucleotide polymorphisms (SNPs) in 5,808 contigs [31],[32]。Contigs with the SNPs were searched with blastn againstAe。tauschiigenome sequences [20] and barley genome sequences [33]; these genome sequences included high-confidence genes with anE-value threshold of 10−5and hit length ≥ 50 bp, fingerprinted contigs, and whole genome shotgun assemblies.
To choose scaffolds forAe。tauschiisequences throughout theIw2chromosomal region, all the genes contained in each scaffold were searched with blastn against the barley genomic sequence using parameters described above. Scaffolds containing at least one gene aligned on the distal region of chromosome 2HS (between 3.66 Mb and 5.51 Mb) were considered possible candidates for marker development. Scaffolds without genes were anchored based on respective results from the blastn searches against the barley genome. First, high-confidence SNPs [31],[32] plotted in this 2HS chromosomal segment were used for marker development to refine the target region. Next, SciRoKo version 3.4 [34)是用于搜索模式设置”不匹配;fixed penalty” to identify additional SSR markers in sequence data of candidate scaffolds. Additional SNPs were also identified on candidate scaffolds by sequencing approximately 700 bp of amplified DNA of twoAe。tauschiiaccessions, KU-2154 and KU-2126. The nucleotide sequences were determined using an Applied Biosystems 3730xlDNA Analyzer (Applied Biosystems, Foster City, CA, USA), and SNPs were found via sequence alignments constructed and searched with GENETYX-MAC version 12.00 software (Whitehead Institute for Biomedical Research, Cambridge, MA, USA).
For genotyping, total DNA was extracted from leaves taken from each of the 210Ae。tauschiiaccessions and the 17 Iranian wheat landraces. SSR amplification and detection of polymorphisms at these loci were conducted as described above. The identified SNPs were then further developed into cleaved amplified polymorphic sequence (CAPS) or high resolution melting (HRM) markers. The primer sequences for each SNP marker and any relevant restriction enzymes are summarized in Additional file1。PCR and subsequent analyses were performed as described previously [31],[32],[35]。
Blast analysis of the Ae. tauschii genes relative to the Brachypodium genome
Nucleotide sequences and annotation information of the selectedAe。tauschiiscaffolds were analyzed with reference to theAe。tauschiidraft genome data, which was published by Jia et al. [20]。Reference sequences fromBrachypodium[36] were searched against the National Center for Biotechnology Information (NCBI) NR protein database using the blastx algorithm with anE-value cut-off of 10−3。
Association analysis of the linked markers with glaucousness
The Q + K method was conducted using a mixed linear model (MLM) function in TASSEL ver 4.0 software [37] for an association analysis by incorporating phenotypic and genotypic data and information on population structure. In a previous report, the Bayesian clustering approach implemented in the software program STRUCTURE 2.3 [38] was used with the settingk= 2 to predict the population structure of theAe。tauschiiaccessions [19]。The Q-matrix of population membership probabilities was served as covariates in MLM. Kinship (K) was calculated in TASSEL based on the genotyping information of the 169 DArT markers for the 206Ae。tauschiiaccessions [19]。We performed theF-statistics and calculated theP-values for theF-test, and the threshold value was set as 1E-3 for the significant association. We omitted the target markers from the association analysis when their minor allele frequencies were less than 0.05.
Results
Wax production variation among Ae. tauschii accessions and among synthetic wheat lines
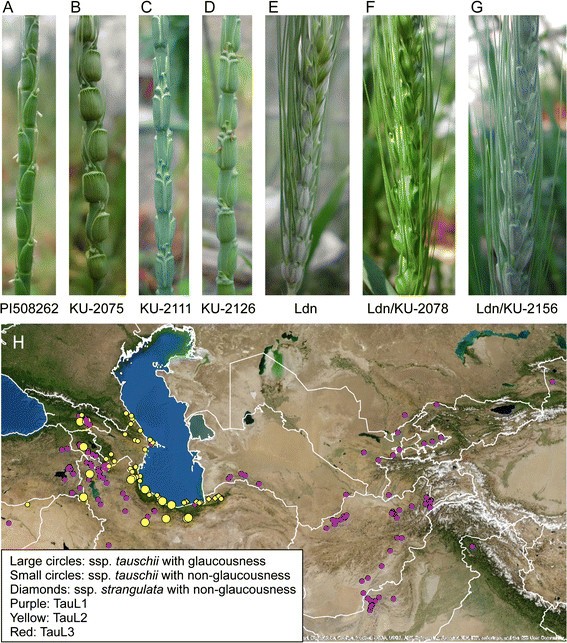
Of the 210Ae。tauschiiaccessions examined, only 20 (9.5%) exhibited the glaucous phenotype and produced whitish wax on the surfaces of peduncles and spikes (Figure1A-D, Additional file2).Wax production for each accession was completely consistent between the Fukui and Kobe environments. Each glaucous accession belonged toAe。tauschiisubspeciestauschii; in other words, none belonged toAe。tauschiisubspeciesstrangulata; the geographic distribution of glaucous accessions was limited to the area that spans from Transcaucasia to the southern coastal region of the Caspian Sea (Figure1H). In the eastern habitats (central Asia, Afghanistan, Pakistan, India, and China) of the species range, no glaucous accession was found. Of the 20 glaucous accessions, 19 belonged to the TauL2 lineage, and only one (IG127015 collected in Armenia) belonged to the TauL1 lineage (Additional file2).
Variation in cuticular wax production amongAe。tauschiiaccessions. (A,B)Non-glaucous accessions ofAe。tauschii。PI508262和ku - 2075被列为subspeciestauschiiand subspeciesstrangulata, respectively.(C,D)Glaucous accessions ofAe。tauschii。(E)A tetraploid wheat cultivar Langdon.(F)A synthetic hexaploid wheat line with the non-glaucous phenotype: the line was derived from an interspecific cross between Langdon and a non-glaucousAe。tauschiiaccession, KU-2078.(G)A synthetic hexaploid wheat line with the glaucous phenotype; the line was derived from an interspecific cross between Langdon and a glaucousAe。tauschiiaccession, KU-2156.(H)Geographical distribution of glaucous-type accessions inAe。tauschii。TheAe。tauschiiaccessions were classified into three genealogical lineages, TauL1, TauL2, and TauL3 [19]。
Of the 82 synthetic wheat lines that we examined, 15 exhibited whitish wax production on the peduncle and spike surface (Figure1E-G), whereas no wax production was evident in any of the 67 other lines (Additional file2).Of the 15 lines that showed the glaucous phenotype, 13 were produced by crossing Ldn with glaucousAe。tauschiiaccessions, and each of the 67 non-glaucous lines was produced by crossing Ldn with a non-glaucousAe。tauschiiaccession. Notably, two synthetic lines, Ldn/KU-2104 and Ldn/KU-2105, exhibited the glaucous phenotype even though their parentalAe。tauschiiaccessions were non-glaucous.
Mapping of the Iw2 locus in Ae. tauschii and synthetic wheat
Two F2populations ofAe。tauschiiand three F2populations from the synthetic wheat lines were analyzed to map the loci that control inhibition of wax production. Each F1plant used for the five cross combinations exhibited the non-glaucous phenotype. In each F2population, the ratio of non-glaucous to glaucous individuals was 3:1; these findings were statistically significant and consistent with Mendelian segregation of alleles of a single gene (Table1).These results indicated that a single genetic locus was associated with the phenotypic difference between non-glaucous and glaucous surfaces on peduncles and spikes, and that allele conferring the non-glaucous phenotype was dominant and the allele conferring the glaucous phenotype was recessive.
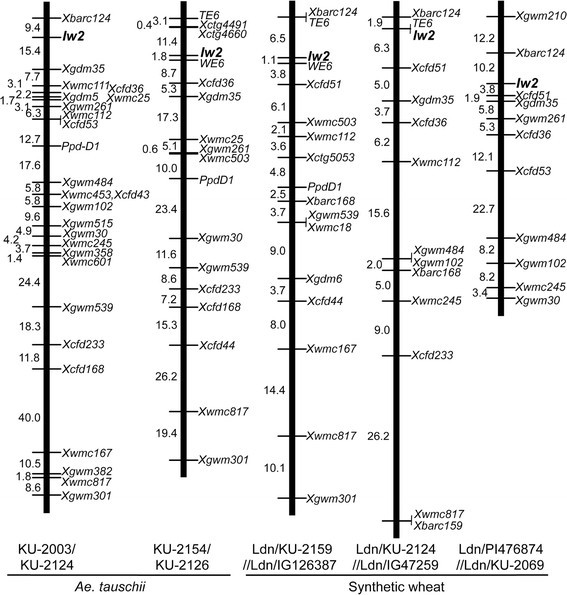
A single locus that controlled inhibition of wax production inAe。tauschiiwas mapped to the same region of the short arm of chromosome 2D in each F2mapping population (Figure2).In the KU-2003/KU-2124 population, the locus that controlled inhibition of wax production, together with the loci for 25 SSR markers andPpd-D1, was assigned to chromosome 2D, and the map length was 230.0 cM with an average inter-loci interval of 8.85 cM. In the KU-2154/KU-2126 population, the locus that controlled inhibition of wax production, together with 14 SSR and 2 STS markers andPpd-D1, was assigned to chromosome 2D, and the map length was 175.4 cM with average inter-loci spacing of 10.32 cM. In the three synthetic wheat populations, Ldn/KU-2159//Ldn/IG126387, Ldn/KU-2124//Ldn/IG47259, and Ldn/PI476874//Ldn/KU-2069, the locus that controlled inhibition of wax production was mapped to a similar position on the short arm of chromosome 2D (Figure2).In these three synthetic wheat populations, the locus that controlled inhibition of wax production was mapped together with 11 to 13 SSR markers, 0 to 2 STS markers, andPpd-D1; additionally, the map lengths ranged from 79.4 to 93.8 cM with an average inter-loci spacing of 4.96 to 8.53 cM.
WE6andTE6是美国东部时间-derived STS markers that are linked toIw2in two mapping populations [7],[9]。In three of our mapping populations, linkage of the non-glaucousness loci toWE6andTE6were confirmed. Thus, the position of one locus that controlled inhibition of wax production inAe。tauschiicorresponded to the well-known wax inhibitor gene,Iw2, on chromosome 2D [6],[7]。Therefore, hereafter, all glaucousness-related loci mapped in this study were considered to be identical toIw2。
Fine mapping of the Iw2 locus
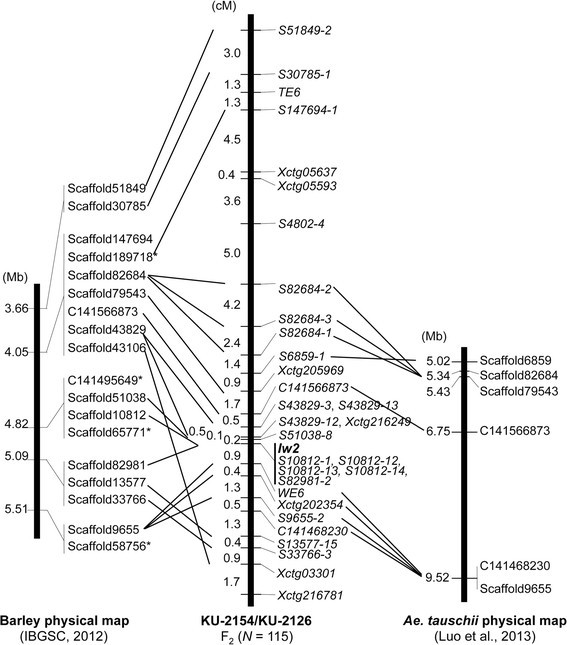
The high-confidence SNPs derived fromAe。tauschiiRNA-seq data have been plotted onto barley chromosomes [32], and physical map information for the barley genome is available [33]。Additionally, physical map information forAe。tauschiiand 16,876 scaffolds that constitute 1.49 Gb from the draftAe。tauschiigenome sequence are anchored to theAe。tauschiilinkage map [20],[21]。The RNA-seq-derived SNP information [31],[32] was used to map seven high-confidence SNPs, represented asXctgloci in Figure3, throughout theIw2chromosomal region in the KU-2154/KU-2126 F2population. Of the sevenXctgloci, four were located within the 8.8 cM chromosomal region immediately surroundingIw2。Nucleotide sequences of the four cDNAs corresponding to theseXctgloci were used as queries to select the carrier scaffolds fromAe。tauschiisequences. We selected theAe。tauschiiscaffolds that mapped near theXctg-carryingAe。tauschiiscaffolds based on synteny between the wheat and barley genomes and the barley physical map [39]。In all, 18Ae。tauschiiscaffolds were assignedin silicoto an area of theAe。tauschiigenome that corresponded to theIw2地区大麦染色体2 h的物理图谱(Figure3).Using a previously developed physical map of theAe。tauschii2DS chromosome [21], we mapped sixAe。tauschiiscaffoldsin silicoto the corresponding region in the 2DS physical map. Nucleotide sequences of the selected scaffolds were used to design CAPS or SSR markers for each scaffold, and the markers that were polymorphic between KU-2154 and KU-2126 were then mapped in the F2population (Figure3).
Comparison of theIw2linkage map, which contains theAe。tauschii支架,物理的大麦和地图Ae。tauschii。TheAe。tauschiiscaffolds were assigned to regions of the barley physical map of chromosome 2H [33]。AnAe。tauschiiphysical map with the mapped scaffolds [21] is represented. Scaffold positions (Mb) and numbers [20],[21] are shown on the left and right of each chromosome, respectively.
Of the selected scaffolds, 23 were mapped to theIw2chromosomal region on 2DS, and the remaining three scaffolds were assigned to other chromosomes. In the KU-2154/KU-2126 population with 115 F2individuals, theIw2locus was mapped within the 1.1 cM interval between the most closely linked markers (Figure3).A dominant marker (S51038-8), derived from theAe。tauschiiscaffold 51038 sequence, was located 0.2 cM distal toIw2, and theWE6SSR marker was located 0.9 cM proximal toIw2。Five co-dominant markers, derived from twoAe。tauschiiscaffolds 10812 and 82981, co-localized withIw2。The marker order in the KU-2154/KU-2126 linkage map was generally conserved with that in the barley 2H physical map. However, barley scaffold 9655 was more closely linked to the barleyIw2ortholog than were two correspondingAe。tauschiiscaffolds, 13577 and 33766, to thetauschii Iw2ortholog; this positioning indicated that a local inversion had occurred in the region proximal toIw2during the divergence between barley andtauschii。
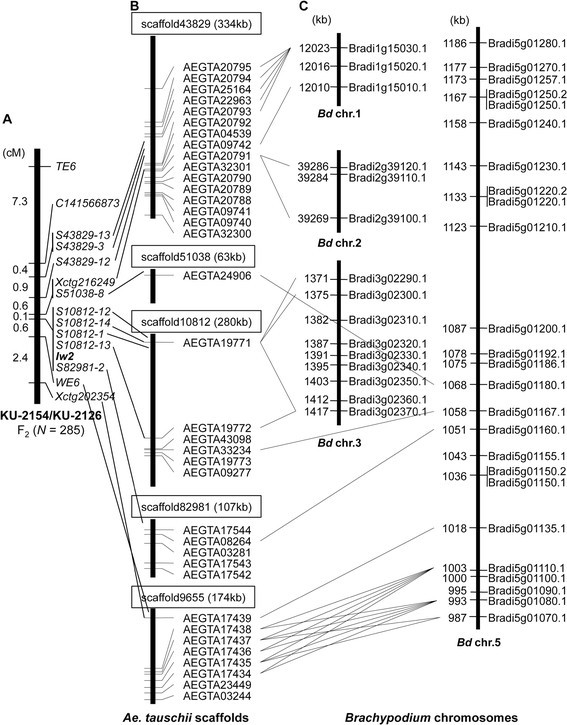
Next, F2individuals of the KU-2154/KU-2126 population and 12 markers from fiveAe。tauschiiscaffolds were used to construct a fine map ofIw2(Figure4A). Based on this linkage map,Iw2was located within the 0.7 cM betweenXctg216249/S51038-8andWE6and co-localized with five markers derived from two scaffolds, 10812 and 82981. Each of the five scaffolds was 63 to 334 kb in length and included one to 16 putative protein-coding genes [20],[21]; marker positions of each scaffold are indicated in Figure4B. Of the 12 markers, eight were derived from intergenic regions, the other four from open reading frames.
Assignment of protein-encoding genes found on the scaffolds aroundIw2to orthologs onBrachypodiumchromosomes. (A)Linkage map of the region aroundIw2generated with 285 F2individuals. Genetic distances (cM) are shown on the left, and markers on the right.(B)The figure shows the positions of putative genes and mapped markers in theAe。tauschiiscaffolds anchored to theIw2region.(C)TheIw2-orthologous regions onBrachypodiumchromosomes based on the blastx search of anchoredAe。tauschiigenes.Brachypodiumgenes are shown on the right, and their position (kb) on the left.
In all, 36 genes were evident on the five scaffolds, and gene annotation could be confirmed for 27 of the 36 genes (Table2).Of these 27Ae。tauschiigenes, 10 putatively encoded cytochrome P450 monooxygenase proteins, and eight encoded disease-related proteins. Additionally, genes encoding laccase, agmatine coumaroyltransferase, receptor kinase, and cell number regulator 2-like were found on the two scaffolds that co-localized withIw2。
TheAe。tauschiiscaffolds that included protein coding genes were used as queries to search theBrachypodiumgenomic information via a blastn search. Of theAe。tauschiigenes on the five scaffolds, 18 had obvious orthologs in theBrachypodiumgenome (Figure4C). Putative orthologs of theAe。tauschiigenes from the four scaffolds were assigned to the 987 to 1068 kb region ofBrachypodium5号染色体。In addition, threeBrachypodiumparalogs (Bradi5g01220.1, Bradi5g01220.2, and Bradi5g01230.1) positioned in the 1133 to 1143 kb region were orthologous to anAe。tauschiigene, AEGTA20985; additionally, Bradi5g01280.1 at 1186 kb was orthologous to AEGTA28084 in scaffold 6859. The locations of twoAe。tauschiigenes, AEGTA20985 and AEGTA28084, were 3 and 3.9 cM, respectively, distal toIw2(Figure3); therefore, the distal part ofIw2showed chromosomal synteny toBrachypodium5号染色体。Thus, theIw2chromosomal region on 2DS was generally syntenic toBrachypodium5号染色体。However, putative orthologs of theAe。tauschiigenes from scaffold 43829 were assigned toBrachypodiumchromosomes 1 and 2. Two paralogousAe。tauschiigenes, AEGTA19771 and AEGTA19772, on scaffold 10812 were orthologous to three paralogousBrachypodiumgenes (Bradi3g02290.1, Bradi3g02300.1, and Bradi3g02370.1) onBrachypodiumchromosome 3. Therefore, the chromosomal synteny betweenAe。tauschiiandBrachypodiumaround theIw2orthologs was complex with regard to chromosome structure.
Iw2-linked marker genotypes in Ae. tauschii
To determine the genetic associations among the developed markers and glaucousness, 13Iw2-linked PCR markers—including five CAPSs, five SSRs, one HRM, one insertion/deletion (indel), and one dominant (presence or absence) marker—were used to genotype the 210Ae。tauschiiaccessions (Table3).For eight of the 13 markers, the 210 accessions exhibited just two apparent alleles; additionally, the set of accessions exhibited just three distinct electrophoresis patterns—including the KU-2154-type, the KU-2126- type, and one other type—at one SSR marker forWE6。The other four SSR markers were highly polymorphic among the accessions; specifically, each marker gave rise to more than three distinct electrophoresis patterns.
The association analysis showed that four SSR markers (S43829-13,S43829-12,S10812-1, andS82981-2), an HRM marker (Xctg216249), the dominant marker (S51038-8), an indel marker (S10812-14), and two CAPS markers (S10812-12, andS10812-13), co-localized withIw2in theAe。tauschiilinkage map, were significantly (P< 1E-3) associated with variation in glaucousness; in contrast, the other three genotyped markers were not significantly associated with variation in glaucousness (Table3).The CAPS markerS43829-3was removed from this association analysis because of the low-frequency (<0.05) allele. In particular, the KU-2126-type allele of the SSR locusS10812-1was found only in 15 of the 20 glaucous accessions; moreover, none of the 190 non-glaucous accessions carried this KU-2126-type allele. The other five glaucous accessions carried a third allele of theS10812-1locus. In 55 of the 190 non-glaucous accessions, only four carried the third allele of theS10812-1locus, and the other 135 accessions carried differentS10812-1alleles. Of the four exceptional non-glaucous accessions that carried the thirdS10812-1allele, two were KU-2104 and KU-2105, and these had each been used to generate a synthetic hexaploid wheat line Ldn/KU-2104 and Ldn/KU-2105, respectively; both synthetic lines showed the glaucous phenotype (Additional file2).However, the phenotype of each synthetic hexaploid line (Ldn/KU-2074 and Ldn/KU-2079) derived from the remaining two of the exceptional accessions (KU-2074 and KU-2079) was non-glaucous. Therefore, phenotypic differentiation in glaucousness was almost completely explained by the allelic configuration at theS10812-1locus in these natural populations ofAe。tauschii。
The 17 Iranian wheat landraces showed the KU-2154-type alleles atS43829-3,Xctg216249, andS51038-8, whereas they exhibited the KU-2126-type alleles atC141566873,S10812-12,S10812-14,S10812-13, andXctg202354。In addition, these landraces exhibited various genotypes that differed from the allelic combinations found inAe。tauschiiaccessions atWE6and four SSR marker loci,S43829-13,S10812-1,S82981-2(Table3).AtS43829-12, 15 landraces showed the KU-2126-type genotype, and two exhibited other genotypes.
Discussion
Natural variation for wax production in Ae. tauschii
Glaucousness is presumably among the components of the domestication syndrome in tetraploid wheat [5],[6]。因此,glaucousness显然是一个目标of artificial selection during tetraploid domestication and common wheat speciation; nevertheless, whether glaucousness is an adaptive trait in wild wheat species remains unclear. Cuticular wax on plant surfaces plays an important role in reducing water loss under drought stress conditions forArabidopsisand rice [1],[4], and observations in these other species indicate that relationships between glaucousness and drought stress tolerance are tight. Presence of either theIw1orIw2allele greatly reduces ß-diketones in the wax components of plants, resulting in a non-glaucous phenotype [3],[10]。Comparative study of glaucousness-related genes in near-isogenic lines (NILs) of a common wheat cultivar (S-615) (BC10F3generation; [6]) demonstrates thatIwalleles had a negative impact on drought tolerance [3]。However, another study ofIw1in a NIL (BC2F3generation) of common wheat did not detect an association betweenIw1genotype and water-use efficiency [10]。
In this study, we used a set of 210 accessions that represented the entire geographical range ofAe。tauschiito examine natural variation in wax production amongAe。tauschii, and found 20 glaucous accessions that were collected in the area that spans from Transcaucasus to the southern-eastern coastal region of the Caspian Sea (Figure1, Additional file2).In a previous study of 176Ae。tauschiiaccessions collected from 105 different habitats throughout Afghanistan, Pakistan, and Iran, 17 glaucous accessions were found in this same area that spans from Transcaucasus to the southern-eastern coastal region [16]。Therefore, our findings were fully consistent with previous observations.
Most glaucous accessions belonged to the TauL2 lineage (Additional file2).TauL2 accessions derived from geographically wide-spread sites throughout the Transcaucasus/Middle East region; these sites represented the western habitats ofAe。tauschii[19]。TauL1 accessions were collected from sites widely distributed throughout the species range, and most TauL1 accessions showed a non-glaucous phenotype. Notably, one TauL1 accession (IG127015), collected in Armenia, showed a glaucous phenotype, and the collection site was located in the middle of an area where glaucous TauL2 accessions were collected (Figure1).Genotyping data suggested that IG127015 had anIw2chromosomal region that was very similar to theIw2chromosomal region of the glaucous TauL2 accessions. One possible explanation for this observation is that IG127015 acquired theIw2chromosomal region from some glaucous individual of the TauL2 lineage. Such introgression could occur in the natural habitat where IG127015 was originally sampled and in experimental fields where the accession was propagated for several generations. Another explanation is that IG127015 became a wax producer through ade novorecessive mutation at theIw2locus; this scenario, however, is unlikely because the molecular marker genotypes in theIw2chromosomal region of IG127015 were largely identical to those in theIw2chromosomal region of glaucous TauL2 accessions (Table3).
Whether the glaucous phenotype of the exceptional TauL1 accession was due to introgression of a glaucous allele from a glaucous TauL2 plant may be difficult to discern. Genome-wide marker analyses using SNP array and diversity arrays technology (DArT) systems indicated that TauL2 was clearly distinct and genetically differentiated from TauL1 [18],[19]。This high level of differentiation indicates that the two genealogical lineages have been reproductively isolated, and that, under natural conditions, inter-lineage hybridization seems to have occurred only rarely [17],[18]。Nevertheless, the presence of a glaucous-type TauL1 accession indicated that the hybridization between TauL1 and TauL2 might have occurred, but the number of hybridizations seems to be quite small. Further detailed study is required to clarify the past occurrence of the TauL1-TauL2 inter-lineage hybridization inAe。tauschii.
Causal loci for variation in glaucousness among Ae. tauschii
Previous studies show that, inAe。tauschii, the causal gene for the glaucous/non-glaucous phenotypic difference isIw2, and that the genotypes of glaucous and non-glaucous accessions wereW2W2iw2iw2andW2W2Iw2Iw2, respectively [5],[14]。The molecular markers tightly linked toIw2were very closely associated with glaucous versus non-glaucous phenotypic difference among the 210 accessions ofAe。tauschii(Table3).Thus, the allelic difference at theIw2locus was the main contributor to the phenotypic difference between the glaucous and non-glaucous accessions ofAe。tauschii(Figure2, Table3).In common wheat, the markers derived from Bradi5g01180 and Bradi5g01160 are tightly linked toIw2as well asIw1[10],[11]。Because the loci that control the glaucous versus non-glaucous phenotypic difference inAe。tauschiimapped to the chromosome 2DS region where the common wheatIw2gene resides (Figure4), the sameIw2gene is likely involved in wax production in bothAe。tauschiiand common wheat. Actually, although most SSR markers around theIw2region were highly polymorphic among theAe。tauschiiaccessions and Iranian wheat landraces, three markers co-localized withIw2inAe。tauschii S10812-12,S10812-14, andS10812-13; notably, each showed the KU-2126-type alleles in each of the 17 Iranian wheat landraces (Table3).These results indicated that the Iranian wheat landraces, which exhibited the glaucous phenotype, had theiw2iw2genotype.
Marker order and gene order aroundAe。tauschii Iw2was well conserved with those on barley chromosome 2HS andBrachypodiumchromosome 5 (Figures3and4).相似的染色体之间的同线性Iw1region on 2BS andBrachypodiumchromosome 5 was recently reported based on mapping with common wheat populations [10],[11]。InAe。tauschii, scaffold information derived from the draft genome data were available for detailed analysis of chromosomal synteny at theIw2region. Chromosomal order of the selected scaffolds atIw2revealed the occurrence of a local inversion during divergence between barley andAe。tauschii(Figure3).Moreover, information of predicted genes in the scaffolds showed that putative translocations occurred during divergence betweenBrachypodiumandAe。tauschii(Figure4).These results also indicated that several gaps existed between theAe。tauschiiscaffolds. Thus, colinearity among barley,BrachypodiumandAe。tauschiiwas observed in theIw2syntenic region, as was reported recently [11], but further screening ofAe。tauschiiBAC clones may be required for construction of the complete physical map atIw2。
In the genotyping analysis withIw2-linked markers, non-glaucous accessions with theIw2Iw2genotype constituted the majority of all 210 accessions (Table3).However, four non-glaucous accessions (KU-2074, KU-2079, KU-2104, and KU-2015) shared a genotype atS10812-1(the most tightly linked marker) with five glaucous accessions (IG127015, KU-2106, KU-2158, KU-2159, KU-2160), indicating that these four non-glaucous accessions may have theiw2iw2genotype in spite of theS10812-1genotypes. In fact, synthetic hexaploids from hybrids between Ldn, which has the glaucous genotype (W1W1iw1iw1) [10], and two of the four non-glaucous accessions, KU-2104 and KU-2105, exhibited the glaucous phenotype (Additional file2).In contrast, the phenotypes of all synthetic hexaploids derived from the KU-2074 and KU-2079, were non-glaucous. Accordingly, KU-2074 and KU-2079 seemed to have theIw2Iw2genotype even though they shared anS10812-1genotype with the five glaucous accessions. Taken together, all this evidence indicated thatIw2was the major gene that controls inhibition of wax production inAe。tauschii。
As yet, no loss-of-function allele has been reported forW2, a major wax-producing gene inAe。tauschii。In common wheat, however, some cultivars such as Chinese Spring and Salmon carry the recessivew2allele [5]。Similarly, non-glaucous-type accessions with thew1recessive allele have been discovered among wild emmer wheat [5]。Whether the recessive loss-of-function mutation occurred at the diploid level (i.e., inAe。tauschii) or at the hexaploid level (i.e., inT. aestivum) is not known. Further studies are required to clarify the details of the genetic mechanism that underlies the wax production inAe。tauschii。
Implication of the Iw2 variation in hexaploid wheat speciation
Based on a comparative genic analysis among common wheat and its ancestral species, Tsunewaki [5] suggested that common wheat, which is hexaploid, is the product of a hybrid cross that took place between a glaucous cultivated emmer wheat with the genotypeW1W1iw1iw1and a glaucous wildAe。tauschiiwith the genotype theW2W2iw2iw2genotype in the mountainous region near the southwestern coastal part of the Caspian Sea. Here, we found that, of 210Ae。tauschiiaccessions, only 20 had the glaucous phenotype (Additional file2) and that a dominant allele at theIw2locus were responsible for expression of the non-glaucous phenotype (Table1).Furthermore, we found that, on the basis of the molecular-marker genotypes in theIw2chromosomal region and the phenotypes of the synthetic common wheat lines, virtually all non-glaucous accessions had theIw2Iw2genotype (Table3, Additional file2).A non-glaucous accession that had theiw2iw2genotype was not found among the 210 accessions. This finding was notable because the double recessivew2w2iw2iw2genotype, if present, would have also caused the non-glaucousness phenotype. The reason for the absence of anyAe。tauschiiaccession with thew2w2 iw2iw2genotype from this collection was not clear, but this fact may indicate that functionalW2alleles confer some adaptive advantage under natural conditions. Taken together, the evidence from this study was consistent with the view that glaucousAe。tauschiiindividuals that had theW2W2iw2iw2genotype were involved in the origin of hexaploid common wheat.
Previous evidence based on isozyme variations and DNA marker polymorphisms is consistent with the hypothesis that the birthplace of hexaploid wheat is within a broad area ranging from Armenia to southwestern Caspian Iran [18],[40]-[42]。The geographic range of the parent populations of glaucousAe。tauschiiaccessions was very consistent with the region postulated in this hypothesis (Figure1).However, theAe。tauschiisubspecies-strangulatahas been postulated to be the D-genome donor of common wheat [43]。Of the 210Ae。tauschiiaccessions that we examined, only 12 accessions have markedly moniliform spikes, and each of these were originally collected in the southeastern coastal Caspian region [25],[26]。Taxonomically, these accessions could be classified asAe。tauschiiCoss. subspeciesstrangulata(Eig) Tzvel. Our data demonstrated that all thesestrangulataaccessions, which were not glaucous, had theIw2Iw2genotype (Figure1).On the assumption that the ancestralAe。tauschiihad theW2W2iw2iw2genotype, this finding may suggest that the southeastern coastal Caspian populations ofAe。tauschiisubspeciesstrangulatado not represent the direct descendants of the ancestral populations that gave rise to hexaploid common wheat.
Conclusions
Analysis of theIw2locus may contribute to improve our understanding of the evolution of hexaploid wheat. Of the 210Ae。tauschiiaccessions, only 20 glaucous accessions were found in the area that spans from Transcaucasia to the southern coastal region of the Caspian Sea. Of the 82 synthetic wheat lines that we examined, 15 were glaucous, and each of the 67 non-glaucous lines was produced by crossing Ldn with a non-glaucousAe。tauschiiaccession. Of the 15 glaucous lines, 13 were produced by crossing Ldn with glaucousAe。tauschiiaccessions. The remained two accessions seemed to have theIw2Iw2genotype according to the genotyping analysis with theIw2-linked markers. Therefore, allelic differences at theIw2locus on the short arm of chromosome 2D were the main contributors to the phenotypic difference between the glaucous and non-glaucous accessions ofAe。tauschii。Some molecular markers, such asS10812-1, closely linked toIw2were significantly associated with variation in glaucousness inAe。tauschii。These results suggest that the D-genome donor of common wheat could have been anyAe。tauschiivariant that carried the recessiveiw2allele.
Availability of supporting data
The data sets supporting the results of this article are included within the article and its supplementary files.
Additional files
Abbreviations
- CAPS:
-
Cleaved amplified polymorphic sequence
- HRM:
-
High resolution melting
- Ldn:
-
Langdon
- SNP:
-
Single nucleotide polymorphism
- SSR:
-
Simple sequence repeat
References
Seo PJ, Lee SB, Suh MC, Park MJ, Go YS, Park CM: The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions inArabidopsis。Plant Cell. 2011, 23: 1138-1152. 10.1105/tpc.111.083485.
Mao B, Cheng Z, Lei C, Xu F, Gao S, Ren Y, Wang J, Zhang X, Wang J, Wu F, Guo X, Liu X, Wu C, Wang H, Wang J: Wax crystal-space leaf2, a rice homologue of WAX2/GL1, is involved in synthesis of leaf cuticular wax. Planta. 2012, 235: 39-52. 10.1007/s00425-011-1481-1.
Zhang Z, Wang W, Li W: Genetic interactions underlying the biosynthesis and inhibition of ß-diketones in wheat and their impact on glaucousness and cuticle permeability.PLoS ONE2013, 8:e54129.,
Zhu X, Xiong L: Putative megaenzyme DWA1 plays essential roles in drought resistance by regulating stress-induced wax deposition in rice. Proc Natl Acad Sci U S A. 2013, 110: 17790-17795. 10.1073/pnas.1316412110.
Tsunewaki K: Comparative gene analysis of common wheat and its ancestral species. II. Waxiness, growth habit and awnedness. Jpn J Bot. 1966, 19: 175-229.
Tsunewaki K, Ebana K: Production of near-isogenic lines of common wheat for glaucousness and genetic basis of this trait clarified by their use. Genes Genet Syst. 1999, 74: 33-41. 10.1266/ggs.74.33.
Liu Q, Ni Z, Peng H, Song W, Liu Z, Sun Q: Molecular mapping of a dominant non-glaucousness gene from synthetic hexaploid wheat (Triticum aestivumL.). Euphytica. 2007, 155: 71-78. 10.1007/s10681-006-9302-5.
Yoshiya K, Watanabe N, Kuboyama T: Genetic mapping of the genes for -non-glaucous phenotype in tetraploid wheat.Euphytica. 2011, 177: 293-297. 10.1007/s10681-010-0283-z.
Okamoto Y, Kajimura T, Ikeda TM, Takumi S: Evidence from principal component analysis for improvement of grain shape- and spikelet morphology-related traits after hexaploid wheat speciation. Genes Genet Syst. 2012, 87: 299-310. 10.1266/ggs.87.299.
Adamski NM, Bush MS, Simmonds J, Tumer AS, Mugford SG, Jones A, Findlay K, Pedentchouk N, Von Wettstein-Knowles P, Uauy C: Theinhibitor of wax 1locus (Iw1) prevents formation of ß- and OH-ß-diketones in wheat cuticular waxes and maps to a sub-cM on chromosome arm 2BS. Plant J. 2013, 74: 989-1002. 10.1111/tpj.12185.
Wu H, Qin J, Han J, Zhao X, Ouyang S, Liang Y, Zhang D, Wang Z, Wu Q, Xie J, Cui Y, Peng H, Sun Q, Liu Z: Comparative high-resolution mapping of the wax inhibitorsIw1andIw2in hexaploid wheat.PLoS ONE2013, 8:e84691.,
Dubcovsky J, Echaide M,实施电击,Rousset M,罗MC, Joppa LR, Dvorak J: Seed-storage-protein loci in RFLP maps of diploid, tetraploid, and hexaploid wheat. Theor Appl Genet. 1997, 90: 247-252.
Wang J, Li W, Wang W: Fine mapping and metabolic and physiological characterization of the glume glaucousness inhibitor locusIw3derived from wild wheat. Theor Appl Genet. 2014, 127: 831-841. 10.1007/s00122-014-2260-8.
Watanabe N, Takesada N, Shibata Y, Ban T: Genetic mapping of the genes for glaucous leaf and tough rachis inAegilops tauschii, the D-genome progenitor of wheat. Euphytica. 2005, 144: 119-123. 10.1007/s10681-005-5193-0.
Simmonds JR, Fish LJ, Leverington-Waite MA, Wang Y, Howell P, Snape JW: Mapping of a gene (Vir) for non-glaucous, viridescent phenotype in bread wheat derived fromTriticum dicoccoides, and its association with yield variation. Euphytica. 2008, 159: 333-341. 10.1007/s10681-007-9514-3.
Kihara H, Tanaka M: Morphological and physiological variation amongAegilops squarrosastrains collected in Pakistan, Afghanistan and Iran. Preslia. 1958, 30: 241-251.
Mizuno N, Yamasaki M, Matsuoka Y, Kawahara T, Takumi S: Population structure of wild wheat D-genome progenitorAegilops tauschiiCoss: implications for intraspecific lineage diversification and evolution of common wheat. Mol Ecol. 2010, 19: 999-1013. 10.1111/j.1365-294X.2010.04537.x.
Wang J, Luo MC, Chen Z, You FM, Wei Y, Zheng Y, Dvorak J:Aegilops tauschiisingle nucleotide polymorphisms shed light on the origins of wheat D-genome genetic diversity and pinpoint the geographic origin of hexaploid wheat. New Phytol. 2013, 198: 925-937. 10.1111/nph.12164.
Matsuoka Y, Nasuda S, Ashida Y, Nitta M, Tsujimoto H, Takumi S, Kawahara T: Genetic basis for spontaneous hybrid genome doubling during allopolyploid speciation of common wheat shown by natural variation analyses of the paternal species.PLoS ONE2013, 8:e68310.,
Jia J, Zhao S, Kong X, Li Y, Zhao G, He W, Appels R, Pfeifer M, Tao Y, Zhang X, Jing R, Zhang C, Ma Y, Gao L, Gao C, Spannagl M, Mayer KFX, Li D, Pan S, Zheng F, Hu Q, Xia X, Li J, Liang Q, Chen J, Wicker T, Gou C, Kuang H, He G, Luo Y, et al: International Wheat Genome Sequencing Consortium, Yang H, Liu X, He Z, Mao L, Wang J:Aegilops tauschiidraft genome sequence reveals a gene repertoire for wheat adaptation. Nature. 2013, 496: 91-95. 10.1038/nature12028.
Luo MC, Gu YQ, You FM, Deal KR, Ma Y, Hu Y, Huo N, Wang Y, Wang J, Chen S, Jorgensen CM, Zhang Y, McGuire PE, Pasternak S, Stein JC, Ware D, Kramer M, McCombie WR, Kianian SF, Martis MM, Mayer KFX, Sehgal SK, Li W, Gill BS, Bevan MW, Šimková H, Doležel J, Weining S, Lazo GR, Anderson OD, et al: A 4-gigabase physical map unlocks the structure and evolution of the complex genome ofAegilops tauschii, the wheat D-genome progenitor. Proc Natl Acad Sci U S A. 2013, 110: 7940-7945. 10.1073/pnas.1219082110.
Takumi S, Koyama K, Fujiwara K, Kobayashi F: Identification of a large deletion in the first intron of theVrn-D1locus, associated with loss of vernalization requirement in wild wheat progenitorAegilops tauschiiCoss. Genes Genet Syst. 2011, 86: 183-195. 10.1266/ggs.86.183.
Matsuoka Y, Takumi S, Kawahara T: Natural variation for fertile triploid F1formation in allohexaploid wheat speciation. Theor Appl Genet. 2007, 115: 509-518. 10.1007/s00122-007-0584-3.
Matsuoka Y, Takumi S, Kawahara T: Flowering time diversification and dispersal in central Eurasian wild wheatAegilops tauschiiCoss: genealogical and ecological framework.PLoS ONE2008, 3:e3138.,
Matsuoka Y, Nishioka E, Kawahara T, Takumi S: Genealogical analysis of subspecies divergence and spikelet-shape diversification in central Eurasian wild wheatAegilops tauschiiCoss. Plant Syst Evol. 2009, 279: 233-244. 10.1007/s00606-009-0159-7.
Takumi S, Naka Y, Morihiro H, Matsuoka Y: Expression of morphological and flowering time variation through allopolyploidization: an empirical study with 27 wheat synthetics and their parentalAegilops tauschiiaccessions. Plant Breed. 2009, 128: 585-590. 10.1111/j.1439-0523.2009.01630.x.
Kajimura T, Murai K, Takumi S: Distinct genetic regulation of flowering time and grain-filling period based on empirical study of D genome diversity in synthetic hexaploid wheat lines. Breed Sci. 2011, 61: 130-141. 10.1270/jsbbs.61.130.
Lander ES, Green P, Abrahamson J: MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987, 1: 174-181. 10.1016/0888-7543(87)90010-3.
Kosambi DD: The estimation of map distance from recombination values. Ann Eugen. 1944, 12: 172-175. 10.1111/j.1469-1809.1943.tb02321.x.
Beales J, Turner A, Griffiths S, Snape JW, Laurie DA: Apseudo-response regulatoris misexpressed in the photoperiod insensitivePpd-D1amutant of wheat (Triticum aestivumL.). Theor Appl Genet. 2007, 115: 721-733. 10.1007/s00122-007-0603-4.
Iehisa JCM, Shimizu A, Sato K, Nasuda S, Takumi S: Discovery of high-confidence single nucleotide polymorphisms from large-scale de novo analysis of leaf transcripts ofAegilops tauschii, a wild wheat progenitor. DNA Res. 2012, 19: 487-497. 10.1093/dnares/dss028.
Iehisa JCM, Shimizu A, Sato K, Nishijima R, Sakaguchi K, Matsuda R, Nasuda S, Takumi S: Genome-wide marker development for the wheat D genome based on single nucleotide polymorphisms identified from transcripts in the wild wheat progenitorAegilops tauschii。Theor Appl Genet. 2014, 127: 261-271. 10.1007/s00122-013-2215-5.
Mayer KF, Waugh R, Brown JW, Schulman A, Langridge P, Platzer M, Fincher GB, Muehlbauer GJ, Sato K, Close TJ, Wise RP, Stein N: A physical, genetic and functional sequence assembly of the barley genome. Nature. 2012, 491: 711-716.
Kofler R, Schlötterer C, Lelley T: SciRoKo: a new tool for whole genome microsatellite search and investigation. Bioinformatics. 2007, 23: 1683-1685. 10.1093/bioinformatics/btm157.
Matsuda R, Iehisa JCM, Takumi S: Application of real-time PCR-based SNP detection for mapping ofNet2, a causal D-genome gene for hybrid necrosis in interspecific crosses between tetraploid wheat andAegilops tauschii。Genes Genet Syst. 2012, 87: 137-143. 10.1266/ggs.87.137.
Genome sequencing and analysis of the model grassBrachypodium distachyon。Nature. 2010, 463: 763-768. 10.1038/nature08747.
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES: TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007, 23: 2633-2635. 10.1093/bioinformatics/btm308.
Pritchard JK, Stephens M, Donnelly P: Inference of population structure using multilocus genotype data. Genetics. 2000, 155: 945-959.
Mayer KFX, Martis M, Hedley PE, Simková H, Liu H, Morris JA, Steuernagel B, Taudien S, Roessner S, Gundlach H, Kubaláková M, Suchánková P, Murat F, Felder M, Nussbaumer T, Graner A, Salse J, Endo T, Sakai H, Tanaka T, Itoh T, Sato K, Platzer M, Matsumoto T, Scholz U, Dolezel J, Waugh R, Stein N: Unlocking the barley genome by chromosomal and comparative genomics. Plant Cell. 2011, 23: 1249-1263. 10.1105/tpc.110.082537.
Nakai Y: Isozyme variations inAegilopsandTriticum, IV. The origin of the common wheats revealed from the study on esterase isozymes in synthesized hexaploid wheats. Jpn J Genet. 1979, 54: 175-189. 10.1266/jjg.54.175.
Nishikawa K, Furuta Y, Wada T: Genetic studies on alpha-amylase isozymes in wheat. III. Intraspecific variation inAegilops squarrosaand birthplace of hexaploid wheat. Jpn J Genet. 1980, 55: 325-336. 10.1266/jjg.55.325.
Dvorak J, Luo MC, Yang ZL, Zhang HB: The structure of theAegilops tauschiigenepool and the evolution of hexaploid wheat. Theor Appl Genet. 1998, 97: 657-670. 10.1007/s001220050942.
Jaaska V: Aspartate aminotransferase and alcohol dehydrogenase isoenzymes: intraspecific differentiation inAegilops tauschiiand the origin of the D genome polyploids in the wheat group. Plant Syst Evol. 1981, 137: 259-273. 10.1007/BF00982790.
Acknowledgements
The authors thank Drs. Koichiro Tsunewaki and Kentaro Yoshida for helpful discussions. We are grateful to Dr. Hisashi Tsujimoto for his supplying some genotyping data of the DArT markers. This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid for Scientific Research (B) No. 25292008 to ST.
Author information
Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RN carried out mapping and genotyping experiments and drafted the manuscript. JCMI contributed to the bioinformatics-related analysis. YM surveyed the natural variation inAe。tauschiiand revised the manuscript. ST conceived the study, acquired the funding, examined the variation in wheat synthetics, and drafted and revised the manuscript. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
图片或其他第三方的材料在这rticle are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visithttps://creativecommons.org/licenses/by/4.0/。
The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nishijima, R., Iehisa, J.C.M., Matsuoka, Y.et al.表皮蜡抑制剂轨迹Iw2in wild diploid wheatAegilops tauschii: phenotypic survey, genetic analysis, and implications for the evolution of common wheat.BMC Plant Biol14,246 (2014). https://doi.org/10.1186/s12870-014-0246-y
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/s12870-014-0246-y
Keywords
- Allopolyploid speciation
- Cuticluar wax inhibitor
- Synthetic wheat
- Wheat evolution