- Research article
- Open Access
- Published:
Insights into the evolution and diversification of theAT-hook Motif Nuclear Localizedgene family in land plants
BMC Plant Biologyvolume14, Article number:266(2014)
Abstract
Background
Members of the ancient land-plant-specific transcription factorAT-Hook Motif Nuclear Localized(AHL) gene family regulate various biological processes. However, the relationships among theAHLgenes, as well as their evolutionary history, still remain unexplored.
Results
We analyzed over 500AHLgenes from 19 land plant species, ranging from the early divergingPhyscomitrella patensand年代elaginellato a variety of monocot and dicot flowering plants. We classified the AHL proteins into three types (Type-I/-II/-III) based on the number and composition of their functional domains, the AT-hook motif(s) and PPC domain. We further inferred their phylogenies via Bayesian inference analysis and predicted gene gain/loss events throughout their diversification. Our analyses suggested that theAHLgene family emerged in embryophytes and further evolved into two distinct clades, with Type-IAHLs forming one clade (Clade-A), and the other two types together diversifying in another (Clade-B). The twoAHLclades likely diverged before the separation ofPhyscomitrella patensfrom the vascular plant lineage. In angiosperms, Clade-AAHLs expanded into 5 subfamilies; while, the ones in Clade-B expanded into 4 subfamilies. Examination of their expression patterns suggests that theAHLs within each clade share similar expression patterns with each other; however,AHLs in one monophyletic clade exhibit distinct expression patterns from the ones in the other clade. Over-expression of aGlycine maxAHL PPC domain inArabidopsis thalianarecapitulates the phenotype observed when over-expressing itsArabidopsis thalianacounterpart. This result suggests that theAHLgenes from different land plant species may share conserved functions in regulating plant growth and development. Our study further suggests that such functional conservation may be due to conserved physical interactions among the PPC domains of AHL proteins.
Conclusions
Our analyses reveal a possible evolutionary scenario for theAHLgene family in land plants, which will facilitate the design of new studies probing their biological functions. Manipulating theAHLgenes has been suggested to have tremendous effects in agriculture through increased seedling establishment, enhanced plant biomass and improved plant immunity. The information gleaned from this study, in turn, has the potential to be utilized to further improve crop production.
Background
Genes that regulated essential biological processes in ancient plant species constituted a conserved "gene tool kit", which tended to be preserved throughout evolution [1]-[4]. Most of the members in this "tool kit" have generally duplicated and expanded into multi-member-containing gene families with divergent functions in modern land plants [1],[5],[6]. Understanding their functions as well as evolutionary histories have greatly enhanced our knowledge of plant growth and development, such as the cases of the cytochrome P450s [7], MADS-box transcription factors [8]-[12],AP2/EREBPgenes [13]-[16], theTALEhomeobox gene family [17]-[19], NAC transcription factors [20]-[22],HD-ZIPgenes [23]-[25],Basic/Helix-Loop-Helixgenes [26]-[28] and theTCPgene family [29]-[31].
However, there are also many gene families that are important to land plant evolution whose functions and evolutionary histories are not well understood. The ancient transcription factorAT-Hook Motif Nuclear Localized(AHL) gene family has been found in all sequenced plant species, ranging from the mossPhyscomitrella patens, to flowering plants, such asArabidopsis thaliana,年代orghum bicolor,Zea maysandPopulus trichocarpa.High conservation of this gene family throughout land plant evolution suggests that it is important for plant growth and development. Currently we are beginning to understand the biological functions of severalAHLs. The evolutionary history of this gene family, however, has still barely been explored.
Members of the AHL proteins contain two conserved structural units, the AT-hook motif and thePlant andProkaryoteConserved (PPC) domain, the latter being also annotated as theDomain ofUnknownFunction #296(DUF296) [32]. Since the functions of this domain have been partially revealed [33], hereafter, we will refer it only as the PPC domain. The AT-hook motif enables binding to AT-rich DNA and has been identified in various gene families both in prokaryotes and eukaryotes, including theHighMobilityGroupA(HMGA) proteins in mammals [34]. The AT-hook motif uses a conserved palindromic core sequence, Arg-Gly-Arg, to bind to the minor groove of AT-rich B-form DNA. Upon binding with DNA, this core sequence adopts a concave conformation with close proximity to the backbone of the DNA, with both arginine side chains firmly inserting into the minor groove [35].
The second functional unit of the AHL proteins is the PPC domain, which is approximately 120 amino acids in length and exists as a single protein in Bacteria and Archaea [32]. Crystal structures of several bacterial and archaeal PPC proteins suggested that the prokaryotic PPC proteins form a trimer [36],[37]. In land plants, the PPC domain has been identified in AHL proteins where it is located at the carboxyl end relative to the AT-hook motif(s) [32]. The PPC domain is responsible for the nuclear localization of the AHL proteins as well as protein-protein interactions among AHL proteins and with other common interactors, such as transcription factors. It may suggest a role in regulating transcriptional activation by the AHL proteins in plants [33].
Members of theAHLfamily regulate diverse aspects of growth and development in plants. Most of the studies are from the analyses ofArabidopsis thaliana.年代everalAHLs are suggested to regulate the homeostasis of phytohormones, especially gibberellins [38], jasmonic acid [39] and cytokinins [40]. Two members of theArabidopsis thaliana AHLgene family,年代UPPRESSOR OF PHYTOCHROME B-4 #3(年代OB3/AHL29) andESCAROLA(ESC/AHL27), repress hypocotyl elongation for seedlings grown in the light [41]. As adults, theAtAHLover-expression plants develop enlarged organs, such as expanded leaves, flowers and fruits as well as delayed flowering and senescence [41]. Similar functions have also been proposed forAtAHL22, andHERCULES(HRC/AHL25) [42],[43].Arabidopsis thaliana ESC/AtAHL27andAHL20have also been implicated in the regulation of plant defense responses [44],[45].
In this study, we identified members of theAHLgene family in the completely sequenced genomes of 19 land plant species, ranging from the mossPhyscomitrella patensand the lycophyte年代elaginellato a variety of monocot and dicot species in the Phytozome database [46]. A closer look at their protein sequences revealed that these land plant AHL proteins can be divided into three types (Type-I, -II and -III) based on a combination of the number and composition of its two structural units, the AT-hook motif(s) and the PPC domain. The Type-IAHLs form one clade; while the Type-II and -IIIAHL在一起形成一个单独的分支。系统发育安娜lysis of theAHLgenes in basal plants suggests that such divergence between the two clades dated between the appearance of chlorophytes and mosses. In this study, we have further identified that theAHLgene family in land plants evolved into 9 phylogenetic sub-families. Finally, we have proposed an evolutionary scenario for theAHLgene family in land plants.
Results
Early divergence in the land-plant AHL protein family
Members of theAHLgene family contain two functional units, the AT-hook motif and the PPC domain [32]. In order to identify theAHLgenes in land plant species, we performed searches against the Phytozome database using theAHLnucleotide and amino acid sequences fromArabidopsis thaliana[46]. We further added the retrieved results as additional queries to perform further searches to identifyAHLgenes from the genomes of 19 plant species (Figure1a, Additional files1,2and3).
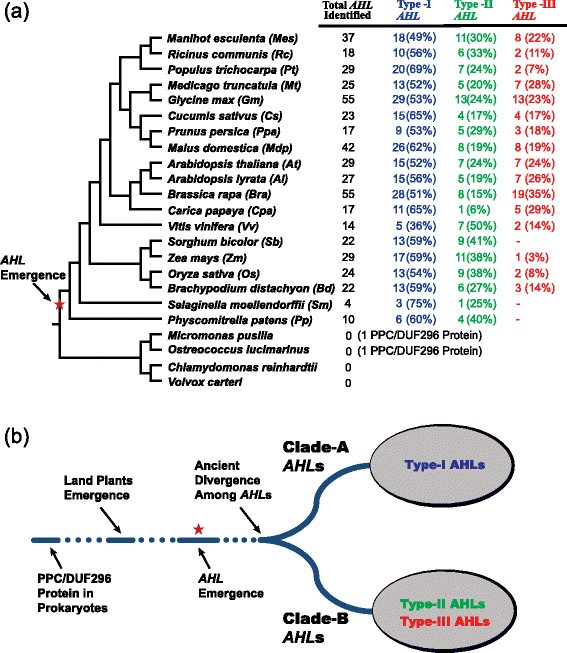
AHLgenes identified in land plant species. (a)The numbers of theAHLgenes identified in each sequenced plant genome were listed accordingly. The percentages of each type were also listed in parenthesis.(b)AHLgenes emerged in land plant species and further diverged into two separate monophyletic clades (Clade-A and Clade-B). The red star denoted the time point when theAHLgenes are likely to have emerged.
Initial phylogenetic analysis of the retrieved AHL proteins in this study suggested that all of the land-plant AHL proteins evolved into two major clades (Figure1b). This distinct division into two monophyletic clades could also be observed in phylogenetic analysis when using just theAHLgenes fromArabidopsis thaliana[32],[33],[38],[41] andOryza sativa[47]. Analysis of all theAHLgenes identified in this study in the moss and lycophytes reveals a similar distribution into these two clades. This further suggests that the division between these two branches dated before the divergence of mosses from the rest of the land plants.
Each monophyletic clade defines one type of PPC domain in land plant AHL proteins
Examination of the PPC domains revealed that their protein sequences share unique characteristics within each of the two AHL phylogenetic clades (Figure1b, Additional file4).The Clade-A AHL proteins share the same type of PPC domain (hereby named "Type-A PPC domain"). Clade-B AHL proteins share another type of PPC domain (hereby named "Type-B PPC domain").
In order to further examine the divergence between the PPC domains in AHL proteins, we performed a sequence logo analysis. The Type-A PPC Domain in Clade-A generally starts with Leu-Arg-Ser-His (Additional file4a); while the Type-B PPC domain in Clade-B generally starts with Phe-Thr-Pro-His (Additional file4b). Both types of PPC domains in AHL proteins are further followed by stretches of amino acid residues with moderate conservation. Examination of both types of PPC domains in the identified AHL proteins revealed that they contain a consensus conserved Gly-Arg-Phe-Glu-Ile-Leu motif (Additional file4a, b). It is also interesting to note that the coding sequences of this motif always exists at the immediate beginning of one exon region in the intron-containing Type-B PPC/DUC296 domains. The sequence upstream of the conserved six amino acids in Type-B PPC domains is generally Thr-Tyr-Glu, while it is generally Thr-Lys-His upstream of the six amino acids in Type-A PPC domains. The sequences downstream of the conserved six amino acids in both types of PPC domains are similar to each other.
Conserved functions of PPC domains in AHL proteins in land plants
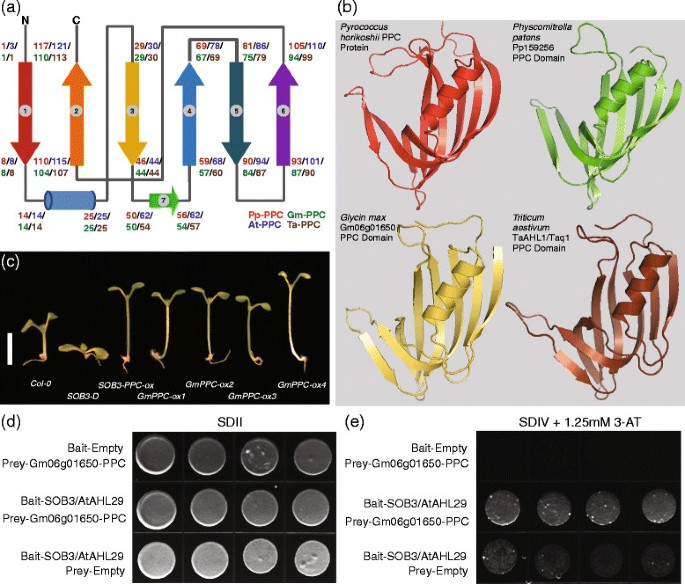
In order to understand the biological functions of the PPC domains in the AHL proteins, we cloned two full-lengthAHLgenes from the bread wheatTriticum aestivumand one PPC domain from a soybeanGlycine max AHLgene (Gm06g01650.1) (Additional file5).AlthoughGm06g01650.1is only a partial gene, it together with the cloned wheatAHLs and twoArabidopsis thaliana AHLs encode proteins that all contain a Type-I AT-hook motif and a Type-A PPC domain (Additional files5and6).They share the same arrangement of secondary structural elements and tertiary structures with each other, as well as with their counterparts in prokaryotes and the moss,Physcomitrella patens(Figure2a and2b). A careful examination reveals that their PPC domains all exhibit aβ1-α-β3-β7-β4-β5-β6-β2secondary structural arrangement, suggesting possible conserved biological functions of this domain among multiple species.
The AHL proteins comprise AT-hook motif(s) and PPC domain. (a)Topology of secondary structures of the AHL PPC domains from multiple land plant species. The cylinder denotes an α-helix and the arrows denote β-sheets. The numbers represent positions of the amino acids in the AHL PPC domain at the corresponding secondary structure positions. Pp-PPC, Pp159256 PPC domain. At-PPC, AtAHL29 PPC domain. Gm-PPC, Gm06g01650.1 PPC domain. Ta-PPC, TaAHL1 PPC domain.(b)Predicted tertiary structures of the PPC domains from these AHL proteins.(c)Hypocotyl growth ofCol-0,年代OB3-D,年代OB3-PPCoverexpression and multipleGm06g01650-PPCoverexpression lines, growing in 20 μmol∙s-1-m-2white light. Scale bar = 5 mm.(d and e)Full length Arabidopsis thaliana SOB3/AtAHL29 interacts with the PPC domain ofGlycine maxGm06g01650.1 in an yeast two-hybrid assay.
To test the hypothesis that the PPC domain may share conserved biological regulatory functions, we overexpressed this domain fromGm06g01650.1driven by the35Sconstitutive promoter in wild-typeArabidopsis thaliana.Multiple homozygous over-expression lines containing single-locus insertions exhibited longer hypocotyls in white light comparing with wild-type controls (Figure2c). This long-hypocotyl phenotype is similar to the one demonstrated by seedlings over-expressing the PPC domain fromArabidopsis thalianaAtAHL29/SOB3 [33], suggesting that shared conserved biological functions exist betweenGlycine maxandArabidopsis thaliana AHLs.
Arabidopsis thaliana AHLs have been suggested to suppress hypocotyl growth in the light [33],[41]. Therefore, the long-hypocotyl phenotype exhibited by over-expressing theGm06g01650.1PPC domain may be conferred through the disturbance of the growth suppression roles ofArabidopsis thaliana AHLgenes. To test this hypothesis, we examined if the PPC domain of Gm06g01650.1 can physically interact with theArabidopsis thalianaAHL proteins using a targetedlexA-based yeast two-hybrid assay (Figure2d,e). Using 1.25 mm 3-amino-1, 2, 4-triazol that prevented transcriptional auto-activation by SOB3/AtAHL29 in the bait protein, we demonstrated that SOB3/AtAHL29 fromArabidopsis thalianaand the PPC domain ofGlycine maxGm06g01650.1 can interact with each other (Figure2d,e).
Type-I and -II AT-hook motifs exist in AHL proteins
Two types of AT-hook motifs (Type-I and -II) are found in the AHL proteins (Figure3a,b; Additional file7) [33],[34]. Both types of AT-hook motifs in the AHL proteins share the same conserved Arg-Gly-Arg core and use this conserved palindromic core to bind the minor groove of AT-rich B-form DNA [35]. Clade-A AHLs contain only one copy of the Type-I AT-hook motifs; while, in Clade-B, some of the AHLs contain only one copy of the Type-II AT-hook motifs and the rest contain both types of AT-hook motifs.
Type of AHL proteins and their AT-hook motifs in land plants.Ice-Logo analysis of the Type-I AT-hook motifs(a)and Type-II AT-hook motifs(b)in land-plant AHL proteins. The star symbol denotes the core sequence of the AT-hook motif. The conserved sequence downstream of the core sequences in Type-I and Type-II AT-hook motifs were pointed out by the triangle and diamond symbols accordingly.(c)Topology of three types of AHL proteins identified in land plants based on the combination of AT-hook motifs and PPC domain.
A specific consensus sequence, Gly-Ser-Lys-Asn-Lys, was observed at the carboxyl end of the Arg-Gly-Arg core sequence in the Type-I AT-hook motifs (Figure3a, Additional file7a,b). The conservation of these downstream sequences is more significant in the AHLs that only contain this type of AT-hook motif. However, these sequences are more variable in other AHLs that also possess a Type-II AT-hook motif (Additional file7b)。只有短共识氨基酸延伸,参数-Lys-Tyr, could be observed downstream of the conserved Arg-Gly-Arg core sequences of the Type-II AT-hook motifs in clades of both AHLs (Figure3b, Additional file7c,d). The conservation of these downstream sequences is similar among the AHLs in either clade (Additional file7c,d).
Three types of AHL proteins in land plants
Based on a combination of type and number of the AT-hook motif(s) and the PPC domain, all the AHL proteins identified in this study can be further classified into three types (Type-I, -II and -III AHLs) (Figure3c). The Type-I AHL proteins contain one Type-I AT-hook motif and one Type-A PPC domain. The Type-II AHL proteins contain two AT-hook motifs (one additional Type-II AT-hook motif at the N-terminus of the Type-I AT-hook motif) and one Type-B PPC domain. Finally, the Type-III AHL proteins contain one Type-II AT-hook motif and one Type-B PPC domain. Clade-A is comprised of the Type-IAHLgenes, while Clade-B is comprised of the Type-II and -IIIAHLgenes. Both clades haveAHLgenes fromPhyscomitrella patens(moss) forming a sister clade to the rest of the members of the clade, indicating an early divergence between the Type-IAHLs and the other two types ofAHLgenes.
Type-I and -II AHLs found in flowering plants were present in early-diverged land plants
In order to understand the evolutionary origin of theAHLgenes, we also performed searches forAHLgenes in chlorophytes. Neither anyAHLgenes nor genes encoding the PPC domain could be identified in the current release of theChlamydomonas reinhardtiiandVolvox carterigenomes (Figure1a) [46],[48],[49]. Surprisingly, we were able to identify only onePPC基因编码的PPC领域没有n associated AT-hook motif(s) inMicromonas pusilla CCMP1545[50] andOstreococcus lucimarinus[51] (Additional file8).To further examine the presence of thePPCgene in picoeukaryotic species, we further examined the genome of an additional picoeukaryotic strainOstreococcus tauri[52]. Similarly, only a single copy of thePPCgene could be identified (Additional file8).This is similar to the case observed in bacterial and archaeal genomes, where each species contains only onePPCgene which encodes a single protein (Additional file8) [32].
We further examined the genomic sequences of theAHLgenes and found that the Type-II and -IIIAHLgenes generally contain introns, while the Type-IAHLgenes lack introns in their genomic sequences. This suggests that it is likely that the intron-less Type-IAHLgenes in land plants is the ancestral form from which the two intron-containing types are derived. In each species, there are generally more Type-IAHLgenes in number than either of the other two types (Figure1a). Compared to other families, the Poaceae species have a lower percentage of Type-IIIAHLgenes, includingZea mays[53],Oryza sativa[54],[55] andBrachypodium distachyon[56]. Notably, in年代orghum bicolor[57] we could not detect any Type-IIIAHLs (Figure1a). It is likely that the Type-IIIAHLs arose latest since the mossPhyscomitrella patensand lycophyte年代elaginella moellendorffiicontain only Type-I and -IIAHLs (Figure1a).
Plant introns have been suggested to play important roles in regulating the expression of their associated genes through alternative splicing [58]-[60], nonsense-mediated mRNA decay [61], or intron-mediated transcriptional enhancement [62]. In order to understand the biological functions of the introns in Type-II and -IIIAHLs, we extracted the intron sequences fromArabidopsis thaliana AHLs and examined their capabilities to enhance the transcription of their associated genes using the IMEter 2.0 server [63]. The first introns of severalAtAHLs demonstrated at least a moderate ability to enhance the transcription of their genes (Additional file9a-c). Particularly, the first introns inAtAHL4,6and14are predicted to strongly enhance their transcription.
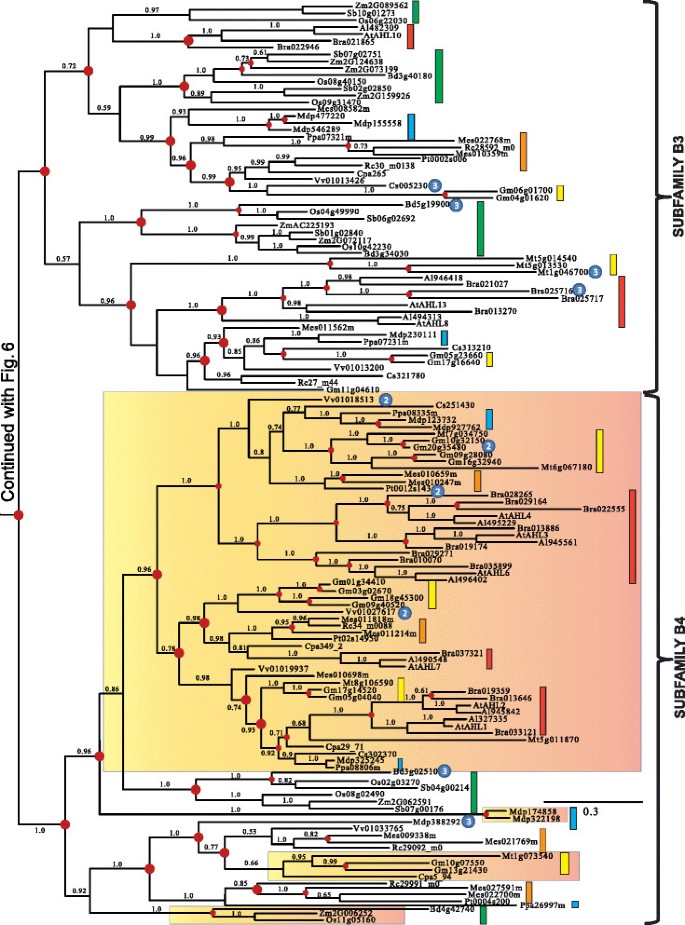
Monophyletic Clade-A contains type-I AHLs
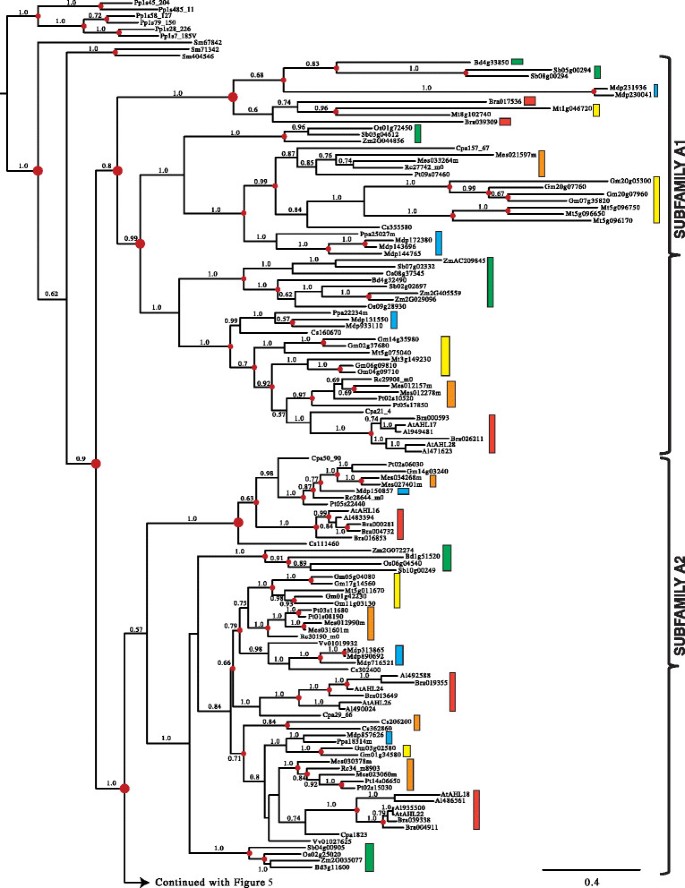
The early divergence between and significant divergence within the twoAHLclades made analyzing them separately necessary to obtain reliable amino acid alignments. We first performed Bayesian inference analysis on the retrieved Clade-AAHLs. The Clade-AAHLs in land plants is comprised of Type-IAHLs that we have organized for discussion convenience into five subfamilies (Subfamilies A1, A2, A3, A4 and A5) (Figures4and5).
Phylogeny of the Clade-AAHLgene family in land plants using Bayesian analysis.Clade-AAHLs are separated into 5 subfamilies (A1, A2, A3, A4 and A5). TwoAHLgenes (TaAHL1andTaAHL3) were cloned fromTriticum aestivumand shown in red. Green boxes representAHLgenes from Poaceae, yellow boxes denote genes from Fabaceae, blue boxes denote genes from Rosaceae, orange boxes denote genes from Malpighiales, and red boxes denote genes from Brassicaceae. Numbers near the branches indicate the Bayesian posterior probabilities for given clades. The red dots at internal nodes denote where gene duplication events have occurred.
Phylogeny of the Clade-AAHLgene family in land plants using Bayesian analysis.Clade-AAHLs are separated into 5 subfamilies (A1, A2, A3, A4 and A5). TwoAHLgenes (TaAHL1andTaAHL3) were cloned fromTriticum aestivumand shown in red. Green boxes representAHLgenes from Poaceae, yellow boxes denote genes from Fabaceae, blue boxes denote genes from Rosaceae, orange boxes denote genes from Malpighiales, and red boxes denote genes from Brassicaceae. Numbers near the branches indicate the Bayesian posterior probabilities for given clades. The red dots at internal nodes denote where gene duplication events have occurred.
In order to better understand the evolutionary events which occurred among these five subfamilies, we reconciled the obtained Bayesian tree with the land-plant species tree and inferred whether the internal nodes within the Clade-A Bayesian tree were associated with gene duplication, gene loss, or lineage divergence events. Since their emergence in land plants, theAHLs within this clade have undergone multiple gene duplication events in the early plant lineages. The Subfamily A1-A5AHLs emerged from lineage divergence events after the divergence of lycophyteAHLs and from the rest of vascular plants and further expanded via a series of gene-duplication/divergence events in angiosperms. The emergence of Subfamily A1, A3 and A5AHLs started via gene-duplication events; while, Subfamily A2 and A4AHLs emerged via speciation events.
Within each subfamily of Clade A,AHLgenes from Euphorbiaceae, Salicaceae, Fabaceae, Rosaceae, Brassicaceae and Poaceae families could all be observed, suggesting they may have evolved from one subfamily-specific most common ancestral gene and later functional divergence occurred among these subfamilies. In the extant plant species, theAHLgenes have undergone extensive gene-duplication/loss events (Table1).The gene duplication events in several extant plant species, such asGlycine max[64] andMalus domestica[65], are probably associated with their recent whole genome duplication events. On the contrary, in several other plant species includingRicinus communis,Carica papaya,Vitis viniferaand monocot species, theAHLgene phylogenies show drastic gene loss events.
Monophyletic Clade-B contains type-II and -III AHLs
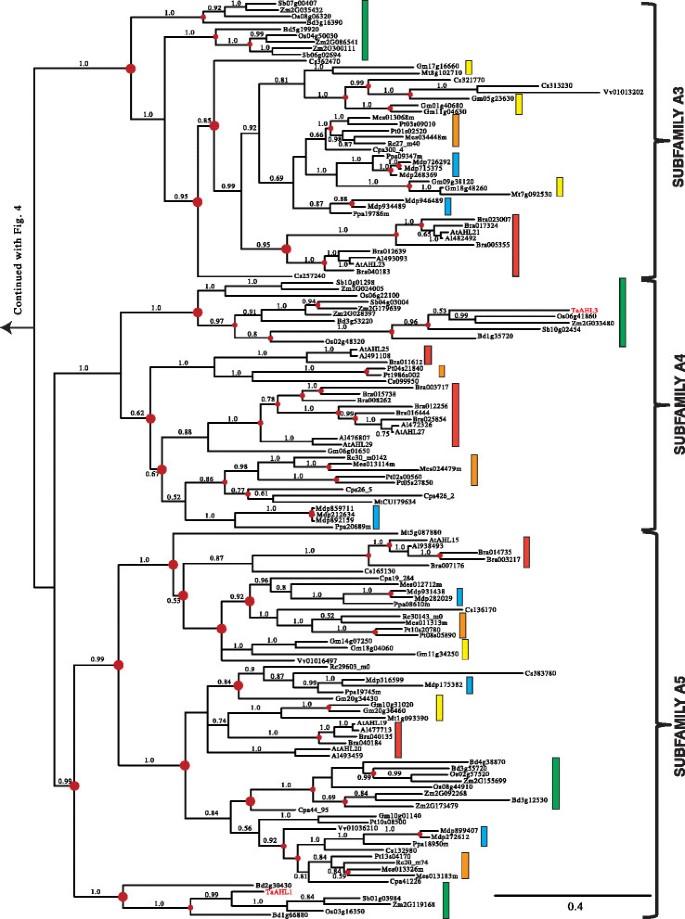
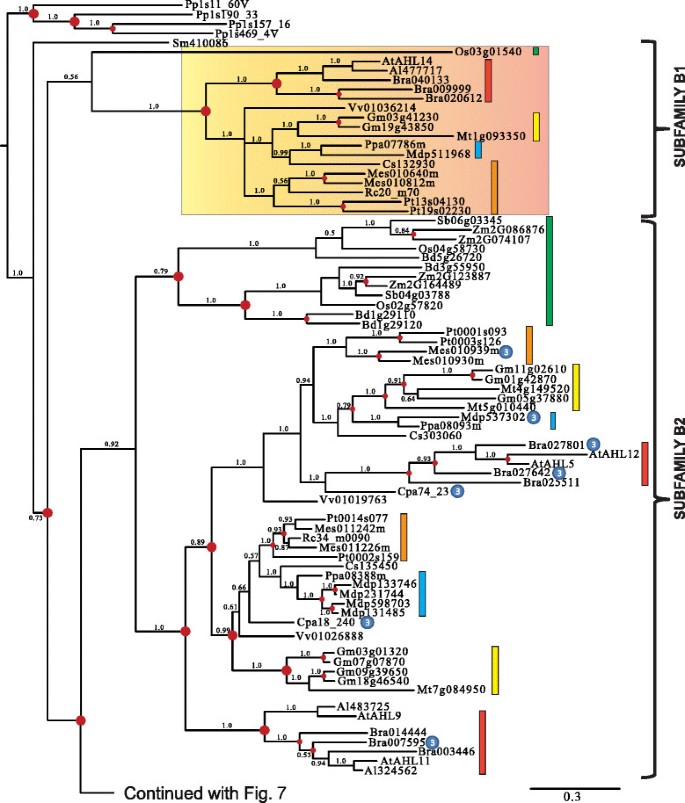
Clade-B of theAHLgene family is comprised of Type-II and Type-IIIAHLs (Figures6and7).The Type-IIAHLs from the early diverging mossPhyscomitrella patensand lycophyte年代elaginella moellendorffiiconstitute a clade at the base of the phylogenetic tree (Figure6).The angiosperm portion of Clade-B can be divided into four subfamilies (Subfamilies B1, B2, B3 and B4).
Phylogeny of the Clade-BAHLgene family in land plants using Bayesian analysis.Clade-BAHLs could be distinguished into 4 subfamilies (B1, B2, B3 and B4). Type-IIIAHLs that form sub-clades have been highlighted by gradient boxes (in Subfamilies B1 and B4) or pointed out by③if within Type-IIAHLsub-clades. Type-IIAHLs are pointed out by②if within a Type-III AHL sub-clades (within Subfamily B4). Green boxes representAHLgenes from Poaceae, yellow boxes denote genes from Fabaceae, blue boxes denote genes from Rosaceae, orange boxes denote genes from Malpighiales, and red boxes denote genes from Brassicaceae. Numbers near branches indicate the Bayesian posterior probabilities for given clades. The red dots at internal nodes denote where gene duplication events have occurred.
Phylogeny of the Clade-BAHLgene family in land plants using Bayesian analysis.Clade-BAHLs could be distinguished into 4 subfamilies (B1, B2, B3 and B4). Type-IIIAHLs that form sub-clades have been highlighted by gradient boxes (in Subfamilies B1 and B4) or pointed out by③if within Type-IIAHLsub-clades. Type-IIAHLs are pointed out by②if within a Type-III AHL sub-clades (within Subfamily B4). Green boxes representAHLgenes from Poaceae, yellow boxes denote genes from Fabaceae, blue boxes denote genes from Rosaceae, orange boxes denote genes from Malpighiales, and red boxes denote genes from Brassicaceae. Numbers near branches indicate the Bayesian posterior probabilities for given clades. The red dots at internal nodes denote where gene duplication events have occurred.
In Subfamilies B1 and B4, members of the Type-IIIAHLs tend to group together and form Type-IIIAHLsub-clades (highlighted with gradient shaded box). Individual members of Type-IIAHLs can be observed within the Subfamily B4 Type-III AHL sub-clades. This indicates possible regaining of the Type-I AT-hook motif within this subfamily, suggesting that not all Type-I AT-hooks are homologous. Individual Type-IIIAHLs also exist within the Type-IIAHLsub-clades (such as Subfamilies B2, B3 and B4). This suggests an independent loss of the Type-I AT-hook motifs by AHL proteins within these subfamilies. Taken together, this indicates there are close evolutionary relationships between these two types ofAHLs with, apparently, multiple transitions from Type-II to Type-IIIAHLs, and from Type-III to Type-IIAHLs. The genomes of the mossPhyscomitrella patensand lycophyte年代elaginella moellendorffiido not contain Type-IIIAHLs, suggesting that the loss of the Type-I AT-hook motif in Clade-B occurred after lycophytes diverged from the rest of vascular plants (Figures1a and6).
年代imilar to their counterparts in Clade A, the Clade BAHLs also experienced multiple gene duplication and loss events during angiosperm diversification (Figures6and7).年代ubfamily B1-B4AHLs emerged from lineage divergence events and further expanded via multiple gene duplication/loss/divergence events (Table1).In each extant plant species, Clade-BAHLs experienced similar numbers of gene duplication/loss events as their counterparts in Clade-A, suggesting shared evolutionary pressure between the two clades.
Members of each AHL monophyletic clade share similar expression patterns
To test the hypothesis that Clade-A and -BAHLs evolved independently, we examined the expression patterns of theAHLs inArabidopsis thalianausing Genevestigator V3 [66]. Based on their expression patterns across various tissues at different developmental stages, the 29Arabidopsis thaliana AHLs can be clearly distinguished into two groups (Additional file10).仔细检查表明,ⅱ型and -IIIAtAHLs tend to share similar expression patterns. Type-II and -IIIAtAHLs, which constitute the Clade-BAHLs, are primarily expressed during seed and flower development. They are only moderately expressed in other tissues. On the other hand, Type-IAtAHLs, which constitute the Clade-AAHLs, are primarily expressed during vascular tissue and root development, which are distinctly different from the expression patterns observed for Type-II and -IIIAHLs. Such distinct expression patterns between the two clades ofAHLs can also be observed inZea mays(Additional file11).
Discussion
TheAHLgene family was first described about 10 years ago, as a group of plant-specific genes encoding proteins containing one or two copies of the AT-hook motif and a 120-amino-acid PPC domain [32]. In this study, AHL proteins have been identified in various plant species, including the early diverging mosses and lycophytes, as well as several angiosperm families [46]. We have further classified the AHL proteins into three types based on the number and composition of these two domains. Accordingly, both the AT-hook motifs and PPC domains of the AHL proteins can be classified into two types based on the phylogenetic analysis performed in this study.
From the prokaryotic PPC proteins to the AHL proteins in land plants
The PPC domain found in the AHL proteins exists by itself as a single protein in prokaryotes [32]. Individual strains of Bacteria and Archaea contain one gene encoding a PPC protein (Additional file8).This observation suggests a role for the PPC domain in fundamental biological processes that has been conserved since prokaryotes throughout evolution. It is intriguing to note that even in the eukaryotic photosynthetic phytoplankton, such asMicromonas pusila[50] andOstreococcus lucimarinus[51), PPC的蛋白质still exists as a single gene. This observation indicates that the association with an AT-hook motif is not necessary for the functions of the PPC protein/domain in prokaryotes and early eukaryotes.
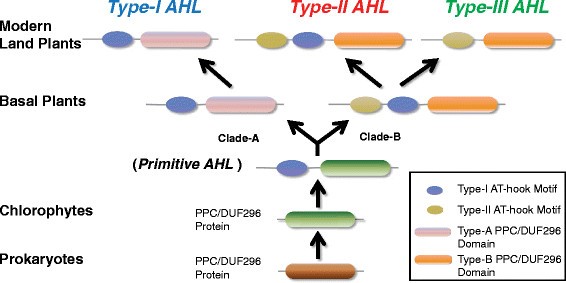
The appearance of the AHL proteins may have occurred between the emergence of the embryophytes and tracheophytes (pointed out by the red star in Figure1a). The primitive AHL proteins emerged when the AT-hook motif fused with the PPC protein between the divergence of picoeukaryotes and the mossPhyscomitrella patens.These primitive proteins later diversified and evolved into two monophyletic clades that comprise the three types of modern AHL proteins found in land plants. However, the evolutionary history of the expansion and later diversifications of theseAHLgenes are yet unexplored.
Ancient events on the AHL evolutionary timeline in land plants
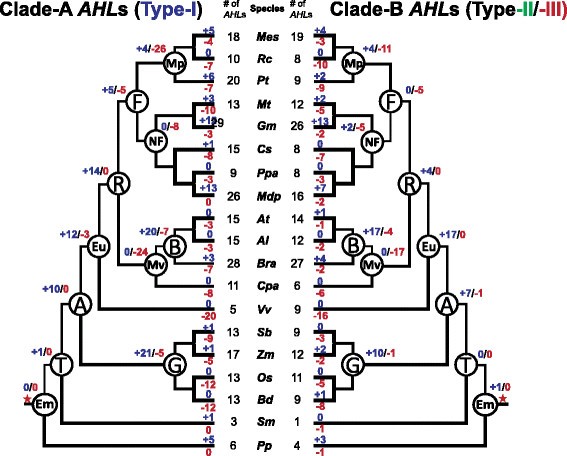
In order to better understand the expansion of the land-plant-specificAHLgenes, we hypothesized the evolutionary events (duplications and deletions) that occurred at common ancestors across land plants (Figure8).In the embryophytes and tracheophytes, there were few gene duplication/loss events occurring after the emergence ofAHLgenes in bothAHLclades. However, both Clade-A and -BAHLs later experienced rapid expansion in angiosperms, which may be responsible for their large numbers in extant angiosperm species. During the emergence of the grass lineage, Clade-AAHLs exhibited more gene duplications than those in Clade-B. However, during the emergence of eudicots, Clade-BAHLs duplicated more rapidly.AHLs in Clade-B expanded in eudicots mainly through numerous gene duplication events; while those in Clade-A were also coupled with a few gene loss events. With the emergence of rosids, Clade-AAHLs duplicated more than their counterparts in Clade-B. Both clades later experienced dramatic gene losses during the emergence of Malvidae (Eurosids II).
Evolutionary events of theAHLgene family in land plants.Numbers of gene duplication (shown in blue after "+") and loss (shown in red after "-") events were inferred for each internal node as well as for current extant species. The numbers of theAHLgenes were also listed accordingly. The red star denotes when theAHLgenes emerged.Al,Arabidopsis lyrata. At,Arabidopsis thaliana. Bd,Brachypodium distachyon. Bra,Brassica rapa. Cpa,Carica papaya. Cs,Cucumis sativus. Gm,Glycine max. Mdp,Malus domestica. Mes,Manihot esculenta. Mt,Medicago truncatula. Os,Oryza sativa. Pp,Physcomitrella patens. Ppa,Prunus persica. Pt,Populus trichocarpa. Rc,Ricinus communis. Sb,年代orghum bicolor. Sm,年代elaginella moellendorffii. Vv,Vitis vinifera. Zm,Zea mays.A, Angiosperms. B, Brassicaceae. Em, Embryophyta. Eu, Eudicots. F, Fabidae (Eurosids I). G, Grasses. Mp, Malpighiales. Mv, Malvidae (Eurosids II). NF, Nitrogen fixing. T, Tracheophyta (vascular plants).
The most dramatic difference between Clade-A and -BAHL年代出现在Fabidae (Eurosid的出现s I). Clade-AAHLs showed rapid birth-and-death events; while the Clade-B copies experienced only gene loss events. This is in direct contrast to theAHLgenes in the emergence of nitrogen fixing species. Clade-AAHLs endured rapid gene losses; while Clade-B copies experienced birth-and-death events. In Malpighiales and Brassicaceae, both clades also emerged through gene birth-and-death events.
A model for the evolutionary history of the AHL gene family in land plants
Based on the results in this study, we propose a model to describe the evolutionary history of theAHLgene family in land plants (Figure9).In this model, thePPCgene existed by itself and encoded a PPC protein in prokaryotes as well as in early Viridiplantae. Prior to the divergence of extant embryophytes, the PPC domain became associated with a Type-I AT-hook motif to form a primitive intron-lessAHLgene. Another Type-II AT-hook motif was further acquired by this type of primitiveAHLbefore the divergence of mosses from the rest of land plants to form a second type ofAHLgene. This new type ofAHLfurther acquired introns in their genomic sequences. The emergence of both types ofAHLs occurred somewhere between the divergence of picoeukaryotes and mosses. These two types of primitiveAHLs duplicated, differentiated and further developed independently into members of Type-I and -IIAHLs in early land plants, defining the two clades (Clade-A and -B). This model is supported by the observation that only these two types ofAHLs could be found in mosses and lycophytes (Figure1).Members of the intron-containing Type-IIAHLs further diversified, some losing the Type-I AT-hook motif while retaining the type-II AT-hook motif, forming the intron-containing Type-IIIAHLs. While we have a general idea of when these events occurred, more detailed sampling among green algae, particularly the streptophyte algae, and more land plant lineages (liverworts, hornworts, ferns, gymnosperms, and monocots other than grasses) is needed to fully resolve the timing of gains and losses of the AT-hook motifs and duplication/deletion events.
Evolution scenario of theAHLgene family in land plants.In prokaryotes and picoeukaryotes, the PPC domain exists by itself as a PPC protein. TheAHLgene family emerged in Embryophytes by incorporating a Type-I AT-hook motif at the N-terminus of the PPC domain, forming primitive Type-I AHL protein(s). TheAHLgenes were further duplicated and gradually evolved into two clades (Clade-B with newly emerged Type-IIAHLs by incorporating one Type-II AT-hook and Clade-A with Type-IAHLs). Along the evolutionary division into the two clades, the PPC domains in theAHLgene family were also evolved into two types. Through the evolution of modern land plants, members of the Type-IIAHLs lost the Type-I AT-hook motif and gradually evolved into the Type-IIIAHLs.
AHL genes belong to the conserved "Gene Tool Kit" in plant evolution
年代ince they originated and diversified early in land plant evolution, theAHLgenes also belong to the conserved "gene tool kit" of ancient plants. Throughout the evolution of land plants, theAHLs co-evolved with other "tool kit" members to regulate essential biological processes. The proposed co-evolution is supported by the observed genetic interactions with other ancient plant gene families, such as the NAC transcription factors and the MADS-box genes [33],[67]. This hypothesis is also supported by the observed physical interactions of AHL proteins with histones (H2B, H3 and H4), TCPs (TCP4, TCP13, and TCP14), ATAF2 and DELLA proteins [33],[68].
The observed physical interactions of the AHL proteins with other non-AHL transcription factors as well as with themselves led to a recently proposed "enhanceosome" molecular model [33]. In this model, it is proposed that the AHL proteins interact with each other to form homo-/hetero-trimer complexes via their PPC domains [33],[69]. A conserved 6-amino-acid motif in the PPC domain from each monomer AHL protein acts together with those from the other two monomers to compose a quaternary domain. This domain in turn may mediate physical interactions with other transcription factors. In this study, over-expression of oneGlycine maxAHL PPC domain recapitulated the long-hypocotyl phenotype reminiscent of over-expressing itsArabidopsis thalianacounterpart (Figure2).This indicates that the AHL PPC domains may serve evolutionarily conserved roles in regulating biological processes in multiple plant species. The AHL proteins share similar secondary and tertiary structures (Figure2).In particular, the 6-amino-acid motif, Gly-Arg-Phe-Glu-Ile-Leu, is highly conserved in the PPC domains of AHLs from all land plants (Additional file4).Therefore, it is possible that the functional conservation of the AHL proteins is achieved through the preservation of interacting partners among different plant species. In this study, we showed thatArabidopsis thalianaSOB3 / AtAHL29可以与之交互Glycine maxGm06g01650.1 PPC domain (Figure2d,e). This observation supports the hypothesis that the AHL proteins from different species can interact with each other via their PPC domains. It would be interesting to test if the preservation of physical interactions between AHL proteins is also conserved among those from more distantly related plant species, such as between AHLs from the mossPhyscomitrella patensand angiosperms, or from monocot and dicot plants. In addition, we have predicted the orthologous and homologousAHLgenes in the examined plant species (Additional files12and13).It would be intriguing to examine if the orthologous/homologous AHLs share similar interactions, genetic and/or physical, with other non-AHL orthologous/homologous partners.
Biological functions of the AHL proteins at AT-rich chromosomal DNA
Besides the potential for shared physical interacting partners, the AHL proteins in the land plant species examined in this study also contain either one copy of Type-I or -II AT-hook motifs or both types. These two types of AT-hook motifs have also been found in the mammalian HMGA proteins [34]. Mammalian HMGA1 binds to AT-rich DNA and serves as an architectural protein which alters the local chromatin state and modulates gene expression through both protein-protein and protein-DNA interactions [70]-[73]. The similar possession of AT-hook motifs by both AHLs and HMGAs suggest that they may share binding affinities for AT-rich DNA.
This association of the AT-hook motif with the PPC domain is likely to physically direct these plant PPC domains to AT-rich chromosomal regions. This notion is supported by the observation thatArabidopsis thalianaAHL1 binds to the AT-rich scaffold/matrix attachment regions (S/MARs) and its AT-hook motif is indispensable for AHL1's DNA binding capacity [32]. The S/MARs have been suggested to primarily localize near the transcription start sites [74],[75] or correlate with the origins of DNA replication [76]. SeveralArabidopsis thalianaAHL proteins bind to gene promoter regions and serve as transcriptional regulators [38],[77]. Therefore, it is likely that the potential targeting of the PPC protein to the S/MARs is correlated with functions in gene transcriptional regulation. It would be interesting to examine and compare the biological functions of both PPC proteins in Bacteria and Archaea with the AHL proteins in land plants in order to shed light on the potential evolutionarily conserved functions of this domain.
In this study, we proposed an evolutionary hypothesis for the diversification ofAHLgenes, from a prokaryotic single-copy gene encoding the PPC protein lacking an AT-hook motif, to three types of land plant AHL proteins incorporating two types of PPC domains and two types of AT-hook motifs. However, the biological functions of these three types of AHL proteins still need to be determined. Further experiments need to be performed to reveal their binding sites along plant chromosomes and the corresponding biological regulatory roles. It should be noted that the inferred evolutionary events in this study are based on the retrieved full-lengthAHLsequences available from current releases of completely sequenced plant genomes. Further analysis should incorporate sequences from additional plant species (particularly ferns and gymnosperms) to improve our understanding of the diversification and functional evolution of the three types of AHL proteins.
Conclusion
In this study, over 500 full-lengthAHLgenes have been identified from 19 fully sequenced plant genomes, ranging from the early divergingPhyscomitrella patensand年代elaginellato a variety of monocot and dicot flowering plants. Our analyses suggest that theAHLs can be classified into three types (Type-I/-II/-III) based on the number and composition of their functional domains, the AT-hook motif(s) and PPC domain. We further inferred their phylogenies in land plants via Bayesian inference analysis. TheAHLgenes emerged in embryophytes and have evolved into two distinct clades with Type-IAHLs diversifying in Clade-A and the other two types together diversifying into Clade-B. Our study indicates that Clade-A and -BAHLs diverged before the separation of mossPhyscomitrella patensfrom the vascular plant lineage. In angiosperms, Clade-AAHLs expanded into 5 subfamilies; while, the ones in Clade-B expanded into 4 subfamilies.
Examination of their expression patterns suggests that theAHLs within each clade share similar patterns of expression with each other. While, theAHLs between the two clades exhibit distinct expression patterns from each other, suggesting potential conserved biological functions within each clade since their divergence along land plant evolution.
Manipulating theAHLgenes has been suggested to have tremendous effects to positively affect agriculture through increasing seedling establishment, plant biomass and improving plant immunity [33],[42],[78],[79]. Our analyses suggest that theAHLgenes from different land plant species may share conserved functions in regulating plant growth and development. Over-expression of aGlycine maxAHL PPC domain inArabidopsis thalianarecapitulates the phenotype observed when over-expressing itsArabidopsis thalianacounterpart. Our study further suggest that such functional conservation may be due to conserved physical interactions among the PPC domains of AHL proteins. In the end, our analyses reveal a possible evolutionary scenario for theAHLgene family in land plants, which will facilitate the design of new studies probing their biological functions and subsequently lead to improvements in crop biomass production.
Methods
Data retrieval
The amino acid sequences as well as coding sequences for the members of theArabidopsis thaliana AHLgene family were retrieved from the TAIR website [32],[41],[80] and were further used as queries for gene search using BLAST, TBLASTN, BLASTP and PSI_BLAST forAHLgenes in the Phytozome database [46] within the related plant species with a cut-off E value set at 1e-2.The obtained results were further used as additional queries. Only intact gene sequences comprised of both AT-hook motif(s) and PPC domain were included and used as additional queries to perform in-depth gene searches in the Phytozome database. For further phylogenetic analysis, only protein sequences were used.
Cloning of AHL genes from Glycine max and Triticum aestivum
Genomic DNA as well as mRNA were prepared fromTriticum aestivumseedlings using DNeasy plant mini kit (Qiagen) and RNeasy plant mini kit (Qiagen). cDNA was further prepared using iScript Advanced cDNA Synthesis Kit for RT-PCR (Bio-RAD). Primer pairs (TaAHL1: 5'-ATG GGG AGC ATG GAC GGC CAC CC-3' and 5'-CTA GAA TGA CGT CGG CGG AGG CCG C-3'; TaAHL3: 5'-ATG GCC ACC GGC AGC AGC AAG TGG TG-3' and 5'-TCA GAT GCC GCC TCC CTG GTG GCC TC-3') were used to cloneTaAHL1andTaAHL3from both prepared genomic DNA and cDNA, correspondingly, and examined to be free-of-introns. Amino acid sequences of TaAHL1 and TaAHL3 proteins were predicted from their coding sequences and were used for further phylogenetic analysis. The nucleotide sequences ofTaAHL1andTaAHL3have been deposited into NCBI GenBank (Accession numbers:TaAHL1/Taq1, KJ461850;TaAHL3/Taq3, KJ461851). Genomic DNA ofGm06g01650.1was prepared from 6 day-old seedlings using ZR Plant DNA MiniPrep kit (Zymo Research). Coding sequence of its PPC domain was cloned using the primer pair (5'-TCC CCC CGG G A TGA AGC CAC CCG TCA TAG TCA CGC GCG AC-3' and 5'-AAC TGC AGT CAA TCA TCA TCA TGC TGA TTC AAG G-3'). The amplicon and binary vectorpCHF3were digested by XmaI with PstI and ligated together. The resulted plasmid was subsequently transformed into agrobacteriumGV3101and further transformed intoArabidopsis thaliana Col-0by the floral dipping method [81]. Surface-sterilized seeds were sown on 0.5× Linsmaier and Skoog modified basal medium (1.0% w/v phytogel and 1.5% w/v sucrose) and grown for 5 days at 25°C under 25 μmol∙s-1-m-2white light in a Percival E-30B growth chamber.
Yeast two-hybrid assay
AlexA-based Y2H system was used to test protein-protein interactions in yeast. The targeted yeast two-hybrid assay was performed as described in [33].
年代equence alignment and phylogenetic analyses
The amino acid sequences of the Type-I AHL proteins were aligned using MUSCLE [82],[83] and were further manually adjusted. Bayesian inference analysis was performed with the MrBayes 3.2.1 on XSEDE tool on CIPRES Science Gateway for 20 million generations with convergence at 0.022 [84]. The amino acid sequences of the Type-II and -III AHL proteins were aligned and manually adjusted. Bayesian inference analysis was performed with the MrBayes 3.2.1 for 10 million generations with convergence at 0.017. Generations were both sampled every 10,000 generations and the first 25% was set as burn-in.
年代econdary and tertiary structure prediction
The amino acid sequences of the PPC domains were retrieved from the coding sequences ofGm06g01650.1, TaAHL1(Taq1) andTaAHL3(Taq3).The secondary and tertiary structures were predicted using the RaptorX Structure Prediction Server [85],[86]. The tertiary structure figures were prepared using Pymol version 1.3 (The PyMOL Molecular Graphics System, Schrodinger, LLC).
Inference of gene duplication and loss event
The plant species tree was adapted from the one in the Phytozome database [46]. The gene trees obtained from Bayesian inference analysis for each of the two AHL clades were reconciled with the plant species tree individually by Notung 2.6 [87] with default parameters. The orthologous and paralogous genes were further inferred by the Notung 2.6 program [87].
Availability of supporting data
The wheatAHLgenes,TaAHL1andTaAHL3were deposited into NCBI GenBank (Accession numbers:TaAHL1/Taq1, KJ461850;TaAHL3/Taq3, KJ461851). All supporting data are included as additional files and have been uploaded to LabArchives, LLC. DOI: 10.6070/H4PC30B2.
Authors' contributions
JZ conceived of the study, participated in its design and coordination, collected the sequences, performed bioinformatics analysis, clonedGm06g01650.1-PPC序列,进行了相关的转基因研究nd wrote the manuscript. DSF performed the yeast two-hybrid analysis and participated in writing the manuscript. JQ clonedTaAHL1/Taq1andTaAHL3/Taq3from wheat. EHR participated in the design of the study, participated in performing the bioinformatics analysis and writing the manuscript. MMN participated in the design and coordination of the study and writing the manuscript. All authors read and approved the final manuscript.
Additional files
Abbreviations
- AHL:
-
AT-hook motif nuclear localized
- PPC/DUF296:
-
Plant and prokaryote conserved/domain of unknown function #296
- 年代OB3:
-
年代uppressor of phytochrome B-4 #3
- ESC:
-
ESCAROLA
- HRC:
-
HERCULES
- Al:
-
Arabidopsis lyrata
- At:
-
Arabidopsis thaliana
- Bd:
-
Brachypodium distachyon
- Bra:
-
Brassica rapa
- Cpa:
-
Carica papaya
- Cs:
-
Cucumis sativus
- Gm:
-
Glycine max
- Mdp:
-
Malus domestica
- Mes:
-
Manihot esculenta
- Mt:
-
Medicago truncatula
- Os:
-
Oryza sativa
- Pp:
-
Physcomitrella patens
- Ppa:
-
Prunus persica
- Pt:
-
Populus trichocarpa
- Rc:
-
Ricinus communis
- 年代b:
-
年代orghum bicolor
- 年代m:
-
年代elaginella moellendorffii
- Vv:
-
Vitis vinifera
- Zm:
-
Zea mays
- A:
-
Angiosperms
- B:
-
Brassicaceae
- Em:
-
Embryophyta
- Eu:
-
Eudicots
- F:
-
Fabidae (Eurosids I)
- G:
-
Grasses
- Mp:
-
Malpighiales
- Mv:
-
Malvidae (Eurosids II)
- NF:
-
Nitrogen fixing
- T:
-
导管植物(维管植物)
References
- 1.
Floyd S, Bowman JL: The ancestral developmental tool kit of land plants. Int J Plant Sci. 2007, 168 (1): 1-35.
- 2.
Tang H, Bowers JE, Wang X, Ming R, Alam M, Paterson AH: Synteny and collinearity in plant genomes. Science. 2008, 320 (5875): 486-488.
- 3.
Ligrone R, Duckett JG, Renzaglia KS: Major transitions in the evolution of early land plants: a bryological perspective. Ann Bot. 2012, 109 (5): 851-871.
- 4.
Pires ND, Dolan L: Morphological evolution in land plants: new designs with old genes. Philos Trans R Soc Lond B Biol Sci. 2012, 367 (1588): 508-518.
- 5.
Lynch M, Conery JS: The evolutionary fate and consequences of duplicate genes. Science. 2000, 290 (5494): 1151-1155.
- 6.
年代terck L, Rombauts S, Vandepoele K, Rouze P, Van de Peer Y: How many genes are there in plants (. and why are they there)?. Curr Opin Plant Biol. 2007, 10 (2): 199-203.
- 7.
Nelson D, Werck-Reichhart D: A P450-centric view of plant evolution. Plant J. 2011, 66 (1): 194-211.
- 8.
Nam J, Kim J, Lee S, An GH, Ma H, Nei MS: Type I MADS-box genes have experienced faster birth-and-death evolution than type II MADS-box genes in angiosperms. Proc Natl Acad Sci U S A. 2004, 101 (7): 1910-1915.
- 9.
Mondragon-Palomino M, Theissen G: MADS about the evolution of orchid flowers. Trends Plant Sci. 2008, 13 (2): 51-59.
- 10.
年代han H, Zahn L, Guindon S, Wall PK, Kong H, Ma H, DePamphilis CW, Leebens-Mack J: Evolution of plant MADS box transcription factors: evidence for shifts in selection associated with early angiosperm diversification and concerted gene duplications. Mol Biol Evol. 2009, 26 (10): 2229-2244.
- 11.
年代maczniak C, Immink RG, Angenent GC, Kaufmann K: Developmental and evolutionary diversity of plant MADS-domain factors: insights from recent studies. Development. 2012, 139 (17): 3081-3098.
- 12.
Jimenez S, Lawton-Rauh AL, Reighard GL, Abbott AG, Bielenberg DG: Phylogenetic analysis and molecular evolution of the dormancy associated MADS-box genes from peach. BMC Plant Biol. 2009, 9: 81-
- 13.
Magnani E, Sjolander K, Hake S: From endonucleases to transcription factors: evolution of the AP2 DNA binding domain in plants. Plant Cell. 2004, 16 (9): 2265-2277.
- 14.
Kim S, Soltis PS, Wall K, Soltis DE: Phylogeny and domain evolution in the APETALA2-like gene family. Mol Biol Evol. 2006, 23 (1): 107-120.
- 15.
年代higyo M, Hasebe M, Ito M: Molecular evolution of the AP2 subfamily. Gene. 2006, 366 (2): 256-265.
- 16.
Rashid M, Guangyuan H, Guangxiao Y, Hussain J, Xu Y: AP2/ERF transcription factor in rice: genome-wide canvas and syntenic relationships between monocots and eudicots. Evol Bioinform Online. 2012, 8: 321-355.
- 17.
Dolan L: Plant evolution: TALES of development. Cell. 2008, 133 (5): 771-773.
- 18.
Lee JH, Lin H, Joo S, Goodenough U: Early sexual origins of homeoprotein heterodimerization and evolution of the plant KNOX/BELL family. Cell. 2008, 133 (5): 829-840.
- 19.
干草,Tsiantis M: KNOX基因:versatile regulators of plant development and diversity. Development. 2010, 137 (19): 3153-3165.
- 20.
Ooka H, Satoh K, Doi K, Nagata T, Otomo Y, Murakami K, Matsubara K, Osato N, Kawai J, Carninci P, Hayashizaki Y, Suzuki K, Kojima K, Takahara Y, Yamanoto K, Kikuchi S: Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2003, 10 (6): 239-247.
- 21.
Zhu T, Nevo E, Sun D, Peng J: Phylogenetic analyses unravel the evolutionary history of NAC proteins in plants. Evolution. 2012, 66 (6): 1833-1848.
- 22.
Hu R, Qi G, Kong Y, Kong D, Gao Q, Zhou G: Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC Plant Biol. 2010, 10: 145-
- 23.
Floyd SK, Zalewski CS, Bowman JL: Evolution of class III homeodomain-leucine zipper genes in streptophytes. Genetics. 2006, 173 (1): 373-388.
- 24.
Prigge MJ, Clark SE: Evolution of the class III HD-Zip gene family in land plants. Evol Dev. 2006, 8 (4): 350-361.
- 25.
Cote CL, Boileau F, Roy V, Ouellet M, Levasseur C, Morency MJ, Cooke JE, Seguin A, MacKay JJ: Gene family structure, expression and functional analysis of HD-Zip III genes in angiosperm and gymnosperm forest trees. BMC Plant Biol. 2010, 10: 273-
- 26.
Toledo-Ortiz G, Huq E, Quail PH: The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell. 2003, 15 (8): 1749-1770.
- 27.
Li X, Duan X, Jiang H, Sun Y, Tang Y, Yuan Z, Guo J, Liang W, Chen L, Yin J, Ma H, Wang J, Zhang D: Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol. 2006, 141 (4): 1167-1184.
- 28.
Pires N, Dolan L: Origin and diversification of basic-helix-loop-helix proteins in plants. Mol Biol Evol. 2010, 27 (4): 862-874.
- 29.
Martin-Trillo M, Cubas P: TCP genes: a family snapshot ten years later. Trends Plant Sci. 2010, 15 (1): 31-39.
- 30.
Mondragon-Palomino M, Trontin C: High time for a roll call: gene duplication and phylogenetic relationships of TCP-like genes in monocots. Ann Bot. 2011, 107 (9): 1533-1544.
- 31.
Preston JC, Hileman LC: Parallel evolution of TCP and B-class genes in Commelinaceae flower bilateral symmetry. Evodevo. 2012, 3: 6-
- 32.
Fujimoto S, Matsunaga S, Yonemura M, Uchiyama S, Azuma T, Fukui K: Identification of a novel plant MAR DNA binding protein localized on chromosomal surfaces. Plant Mol Biol. 2004, 56 (2): 225-239.
- 33.
Zhao J, Favero DS, Peng H, Neff MM: Arabidopsis thaliana AHL family modulates hypocotyl growth redundantly by interacting with each other via the PPC/DUF296 domain. Proc Natl Acad Sci U S A. 2013, 110 (48): E4688-E4697.
- 34.
Aravind L, Landsman D: AT-hook motifs identified in a wide variety of DNA-binding proteins. Nucleic Acids Res. 1998, 26 (19): 4413-4421.
- 35.
Huth JR, Bewley CA, Nissen MS, Evans JN, Reeves R, Gronenborn AM, Clore GM: The solution structure of an HMG-I(Y)-DNA complex defines a new architectural minor groove binding motif. Nat Struct Biol. 1997, 4 (8): 657-665.
- 36.
中村林LY, Nakano H, S,中山教授年代,藤本年代, Matsunaga S, Kobayashi Y, Ohkubo T, Fukui K: Crystal structure ofPyrococcus horikoshiiPPC protein at 1.60 A resolution. Proteins-Struct Func Bioinf. 2007, 67 (2): 505-507.
- 37.
Lin LY, Nakano H, Uchiyama S, Fujimoto S, Matsunaga S, Nakamura S, Kobayashi Y, Ohkubo T, Fukui K: Crystallization and preliminary X-ray crystallographic analysis of a conserved domain in plants and prokaryotes fromPyrococcus horikoshii OT3.Acta Crystallograph Sect F Struct Biol Cryst Commun. 2005, 61: 414-416.
- 38.
Matsushita A, Furumoto T, Ishida S, Takahashi Y: AGF1, an AT-hook protein, is necessary for the negative feedback ofAtGA3ox1encoding GA 3-oxidase. Plant Physiol. 2007, 143 (3): 1152-1162.
- 39.
Endt DV, Silva MSE, Kijne JW, Pasquali G, Memelink J: Identification of a bipartite jasmonate-responsive promoter element in theCatharanthus roseus ORCA3transcription factor gene that interacts specifically with AT-hook DNA-binding proteins. Plant Physiol. 2007, 144 (3): 1680-1689.
- 40.
Rashotte AM, Carson SD, To JP, Kieber JJ: Expression profiling of cytokinin action in Arabidopsis. Plant Physiol. 2003, 132 (4): 1998-2011.
- 41.
年代treet IH, Shah PK, Smith AM, Avery N, Neff MM: The AT-hook-containing proteins SOB3/AHL29 and ESC/AHL27 are negative modulators of hypocotyl growth inArabidopsis.Plant J. 2008, 54 (1): 1-14.
- 42.
江C:修改植物生物量的方法。团结起来d States Patent (US6,717,034 B2). 2004
- 43.
Yun J, Kim YS, Jung JH, Seo PJ, Park CM: The AT-hook motif-containing protein AHL22 regulates flowering initiation by modifyingFLOWERING LOCUS Tchromatin inArabidopsis.J Biol Chem. 2012, 287 (19): 15307-15316.
- 44.
Lim PO, Kim Y, Breeze E, Koo JC, Woo HR, Ryu JS, Park DH, Beynon J, Tabrett A, Buchanan-Wollaston V, Nam HG: Overexpression of a chromatin architecture-controlling AT-hook protein extends leaf longevity and increases the post-harvest storage life of plants. Plant J. 2007, 52 (6): 1140-1153.
- 45.
Lu H, Zou Y, Feng N: Overexpression ofAHL20negatively regulates defenses inArabidopsis.J Int Plant Biol. 2010, 52 (9): 801-808.
- 46.
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS: Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40 (Database issue): D1178-D1186.
- 47.
Kim HB, Oh CJ, Park YC, Lee Y, Choe S, An CS, Choi SB: Comprehensive analysis ofAHLhomologous genes encoding AT-hook motif nuclear localized protein in rice. BMB Rep. 2011, 44 (10): 680-685.
- 48.
Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Marechal-Drouard L, Marshall WF, Qu LH, Nelson DR, Sanderfoot AA, Spalding MH, Kapitonov VV, Ren Q, Ferris P, Lindquist E, Shapiro H, Lucas SM, Grimwood J, Schmutz J, Cardol P, Cerutti H, Chanfreau G, Chen CL, Cognat V, Croft MT, Dent R, et al: TheChlamydomonasgenome reveals the evolution of key animal and plant functions. Science. 2007, 318 (5848): 245-250.
- 49.
Prochnik SE, Umen J, Nedelcu AM, Hallmann A, Miller SM, Nishii I, Ferris P, Kuo A, Mitros T, Fritz-Laylin LK, Hellsten U, Chapman J, Simakov O, Rensing SA, Terry A, Pangilinan J, Kapitonov V, Jurka J, Salamov A, Shapiro H, Schmutz J, Grimwood J, Lindquist E, Lucas S, Grigoriev IV, Schimitt R, Kirk D, Rokhsar DS: Genomic analysis of organismal complexity in the multicellular green algaVolvox carteri.年代cience. 2010, 329 (5988): 223-226.
- 50.
Worden AZ, Lee JH, Mock T, Rouze P, Simmons MP, Aerts AL, Allen AE, Cuvelier ML, Derelle E, Everett MV, Foulon E, Grimwood J, Gundlach H, Henrissat B, Napoli C, McDonald SM, Parker MS, Rombauts S, Salamov A, Von Dassow P, Badger JH, Coutinho PM, Demir E, Dubchak I, Gentemann C, Eikrem W, Gready JE, John U, Lanier W, Lindquist EA, et al: Green evolution and dynamic adaptations revealed by genomes of the marine picoeukaryotesMicromonas.年代cience. 2009, 324 (5924): 268-272.
- 51.
Palenik B, Grimwood J, Aerts A, Rouze P, Salamov A, Putnam N, Dupont C, Jorgensen R, Derelle E, Rombauts S, Zhou K, Otillar R, Merchant SS, Podell S, Gaasterland T, Napoli C, Gendler K, Manuell A, Tai V, Vallon O, Piganeau G, Jancek S, Heijde M, Jabbari K, Bowler C, Lohr M, Robbens S, Werner G, Dubchak I, Pazour GJ, et al: The tiny eukaryoteOstreococcusprovides genomic insights into the paradox of plankton speciation. Proc Natl Acad Sci U S A. 2007, 104 (18): 7705-7710.
- 52.
Derelle E, Ferraz C, Rombauts S, Rouze P, Worden AZ, Robbens S, Partensky F, Degroeve S, Echeynie S, Cooke R, Saeys Y, Wuyts J, Jabbari K, Bowler C, Panaud O, Piegu B, Ball SG, Ral JP, Bouget FY, Piganeau G, De Baets B, Picard A, Delseny M, Demaille J, Van de Peer Y, Moreau H: Genome analysis of the smallest free-living eukaryoteOstreococcus tauriunveils many unique features. Proc Natl Acad Sci U S A. 2006, 103 (31): 11647-11652.
- 53.
年代chnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Liang C, Zhang J, Fulton L, Graves TA, Minx P, Reily AD, Courtney L, Kruchowski SS, Tomlinson C, Strong C, Delehaunty K, Fronick C, Courtney B, Rock SM, Belter E, Du F, Kim K, Abbott RM, Cotton M, Levy A, Marchetto P, Ochoa K, Jackson SM, Gillam B, et al: The B73 maize genome: complexity, diversity, and dynamics. Science. 2009, 326 (5956): 1112-1115.
- 54.
Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, Hadley D, Hutchison D, Martin C, Katagiri F, Lange BM, Moughamer T, Xia Y, Budworth P, Zhong J, Miguel T, Paszkowski U, Zhang S, Colbert M, Sun WL, Chen L, Cooper B, Park S, Wood TC, Mao L, Quail P, et al: A draft sequence of the rice genome (Oryza sativa L. ssp. japonica).年代cience. 2002, 296 (5565): 92-100.
- 55.
Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, Cao M, Liu J, Sun J, Tang J, Chen Y, Huang X, Lin W, Ye C, Tong W, Cong L, Geng J, Han Y, Li L, Li W, Hu G, Li J, Liu Z, Qiu Q, Li T, Wang X, et al: A draft sequence of the rice genome (Oryza sativa L. ssp. indica).年代cience. 2002, 296 (5565): 79-92.
- 56.
Genome sequencing and analysis of the model grassBrachypodium distachyon.Nature. 2010, 463 (7282): 763-768.
- 57.
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, Schmutz J, Spannagl M, Tang H, Wang X, Wicker T, Bharti AK, Chapman J, Feltus FA, Gowik U, Grigoriev IV, Lyons E, Maher CA, Martis M, Narechania A, Otillar RP, Penning BW, Salamov AA, Wang Y, Zhang L, Carpita NC, et al: The年代orghum bicolorgenome and the diversification of grasses. Nature. 2009, 457 (7229): 551-556.
- 58.
Reddy AS, Marquez Y, Kalyna M, Barta A: Complexity of the alternative splicing landscape in plants. Plant Cell. 2013, 25 (10): 3657-3683.
- 59.
年代taiger D, Brown JW: Alternative splicing at the intersection of biological timing, development, and stress responses. Plant Cell. 2013, 25 (10): 3640-3656.
- 60.
Vitulo N, Forcato C, Carpinelli EC, Telatin A, Campagna D, D’Angelo M, Zimbello R, Corso M, Vannozzi A, Bonghi C, Lucchin M, Valle G: A deep survey of alternative splicing in grape reveals changes in the splicing machinery related to tissue, stress condition and genotype. BMC Plant Biol. 2014, 14 (1): 99-
- 61.
Nyiko T, Kerenyi F, Szabadkai L, Benkovics AH, Major P, Sonkoly B, Merai Z, Barta E, Niemiec E, Kufel J, Silhavy D: Plant nonsense-mediated mRNA decay is controlled by different autoregulatory circuits and can be induced by an EJC-like complex. Nucleic Acids Res. 2013, 41 (13): 6715-6728.
- 62.
Morello L, Breviario D: Plant spliceosomal introns: not only cut and paste. Curr Genomics. 2008, 9 (4): 227-238.
- 63.
Parra G, Bradnam K, Rose AB, Korf I: Comparative and functional analysis of intron-mediated enhancement signals reveals conserved features among plants. Nucleic Acids Res. 2011, 39 (13): 5328-5337.
- 64.
年代chmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, Xu D, Hellsten U, May GD, Yu Y, Sakurai T, Umezawa T, Bhattacharyya MK, Sandhu D, Valliyodan B, Lindquist E, Peto M, Grant D, Shu S, Goodstein D, Barry K, Futrell-Griggs M, Abernathy B, Du J, Tian Z, Zhu L, et al: Genome sequence of the palaeopolyploid soybean. Nature. 2010, 463 (7278): 178-183.
- 65.
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, Salvia S, Pindo M, Baldi P, Castelletti S, Cavaiuolo M, Coppola G, Costa F, Cova V, Dal Ri A, Goremykin V, Komjanc M, Longhi S, Magnago P, Malacarne G, Malnoy M, Micheletti D, Moretto M, Perazzolli M, Si-Ammour A, Vezzulli S: The genome of the domesticated apple (Malus x domestica Borkh.).Nat Genet. 2010, 42 (10): 833-839.
- 66.
Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P: Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics. 2008, 2008: 420747-
- 67.
Jin Y, Luo Q, Tong H, Wang A, Cheng Z, Tang J, Li D, Zhao X, Li X, Wan J, Jiao Y, Chu C, Zhu L: An AT-hook gene is required for palea formation and floral organ number control in rice. Dev Biol. 2011, 359 (2): 277-288.
- 68.
Evidence for network evolution in an Arabidopsis interactome map. Science. 2011, 333 (6042): 601-607.
- 69.
Gallavotti A, Malcomber S, Gaines C, Stanfield S, Whipple C, Kellogg E, Schmidt RJ: BARREN STALK FASTIGIATE1 is an AT-hook protein required for the formation of maize ears. Plant Cell. 2011, 23 (5): 1756-1771.
- 70.
Reeves R: Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001, 277 (1-2): 63-81.
- 71.
Lomvardas S, Thanos D: Modifying gene expression programs by altering core promoter chromatin architecture. Cell. 2002, 110 (2): 261-271.
- 72.
Fusco A, Fedele M: Roles of HMGA proteins in cancer. Nat Rev Cancer. 2007, 7 (12): 899-910.
- 73.
Kishi Y, Fujii Y, Hirabayashi Y, Gotoh Y: HMGA regulates the global chromatin state and neurogenic potential in neocortical precursor cells. Nat Neurosci. 2012, 15 (8): 1127-1133.
- 74.
Allen GC, Spiker S, Thompson WF: Use of matrix attachment regions (MARs) to minimize transgene silencing. Plant Mol Biol. 2000, 43 (2-3): 361-376.
- 75.
Pascuzzi PE, Flores-Vergara MA, Lee TJ, Sosinski B, Vaughn MW, Hanley-Bowdoin L, Thompson WF, Allen GC:In vivomapping of arabidopsis scaffold/matrix attachment regions reveals link to nucleosome-disfavoring poly(dA:dT) tracts. Plant Cell. 2014, 26 (1): 102-120.
- 76.
Vaughn JP, Dijkwel PA, Mullenders LH, Hamlin JL: Replication forks are associated with the nuclear matrix. Nucleic Acids Res. 1990, 18 (8): 1965-1969.
- 77.
Franco-Zorrilla JM, Lopez-Vidriero I, Carrasco JL, Godoy M, Vera P, Solano R: DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc Natl Acad Sci U S A. 2014, 111 (6): 2367-2372.
- 78.
Century K, Reuber TL, Ratcliffe OJ: Regulating the regulators: the future prospects for transcription-factor-based agricultural biotechnology products. Plant Physiol. 2008, 147 (1): 20-29.
- 79.
Gonzalez N, Beemster GT, Inze D: David and Goliath: what can the tiny weed Arabidopsis teach us to improve biomass production in crops?. Curr Opin Plant Biol. 2009, 12 (2): 157-164.
- 80.
Huala E, Dickerman AW, Garcia-Hernandez M, Weems D, Reiser L, LaFond F, Hanley D, Kiphart D, Zhuang M, Huang W, Mueller LA, Bhattacharyya D, Bhaya D, Sobral BW, Beavis W, Meinke DW, Town CD, Somerville C, Rhee SY: The Arabidopsis Information Resource (TAIR): a comprehensive database and web-based information retrieval, analysis, and visualization system for a model plant. Nucleic Acids Res. 2001, 29 (1): 102-105.
- 81.
Clough SJ, Bent AF: Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16 (6): 735-743.
- 82.
Edgar RC: MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004, 5: 113-
- 83.
Edgar RC: MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32 (5): 1792-1797.
- 84.
Miller MA, Pferffer W, Schwartz T: Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop. 2010, 14: 1-8.
- 85.
Kallberg M, Margaryan G, Wang S, Ma J, Xu J: RaptorX server: a resource for template-based protein structure modeling. Methods Mol Biol. 2014, 1137: 17-27.
- 86.
Kallberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J: Template-based protein structure modeling using the RaptorX web server. Nat Protoc. 2012, 7 (8): 1511-1522.
- 87.
Chen K, Durand D, Farach-Colton M: NOTUNG: a program for dating gene duplications and optimizing gene family trees. J Comput Biol. 2000, 7 (3-4): 429-447.
Acknowledgements
这个项目是由农业和支持Food Research Initiative competitive grant # 2013-67013-21666 of the USDA National Institute of Food and Agriculture (to M. M. N.), the O.A. Vogel Wheat Research Fund (to M.M.N.) and the Washington Grain Commission (to M. M. N.). This project was also supported by Global Plant Sciences Initiative Research Fellowship (Washington State University, to J. Z.), Pacific Seed Association Fellowship (to J. Z.), Maguire International Seed Technology Fellowship (to J. Z.), Lindahl Memorial Scholarship (to J. Z.) and Roscoe & Francis Cox Scholarship (to J. Z.). We are also grateful for support from the Brubbaken and Reinbold Monocot Breeding Fund (to M. M. N.).
Author information
Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Electronic supplementary material
Chromosomal Locations of
Additional file 1:AHLGenes Identified inArabidopsis thaliana.TheAHLgenes that are resulted from gene duplication were paired with red (adjacent pairs) and blue (distant pairs) lines. (PDF 118 KB)
Chromosomal Locations of
Additional file 2:AHLGenes Identified inOryza sativa.(PDF 138 KB)
年代equence logo analysis of PPC domain of AHL proteins.
Additional file 4: Sequence logo analysis of the Type-A PPC domain(a)and Type-B PPC domain(b)in land-plant AHL proteins. The conserved six-amino-acid region was pointed out by the red boxes. (PDF 129 KB)
12870_2014_266_MOESM5_ESM.pdf
额外的文件5:小麦T的氨基酸序列aAHL1, TaAHL3 and soybean Gm06g01650.1. The AT-hook motif is underlined with green. The PPC domain is underlined with blue. (PDF 111 KB)
Alignment of two
Additional file 6:Arabidopsis thalianaAHLs, AtAHL27 and AtAHL29, with soybean Gm06g01650.1.The AT-hook motif is underlined with green. The PPC domain is underlined with blue. (PDF 66 KB)
年代equence logo analysis of the AT-hook motifs.
Additional file 7: Sequence logo analysis of the Type-I AT-hook motif from(a)the land-plant AHLs that only contain Type-I, not Type-II AT-hook, and from(b)the AHLs that also contain Type-II AT-hook. Sequence logo analysis of the Type-II AT-hook motif in(c)the AHLs that only contain Type-II, not Type-I AT-hook, and(d)the AHLs that also contain Type-I AT-hook. The star symbol represents the core sequence of the AT-hook motif. The conserved sequence downstream of the core sequences in Type-I and Type-II AT-hook motifs were pointed out by the triangle and diamond symbols accordingly. (PDF 96 KB)
Genes Identified in Selected Picoeukaryotes and Prokaryotes.
Additional file 8:PPC/DUF296Genes Identified in Selected Picoeukaryotes and Prokaryotes.(DOC 49 KB)
Intron-mediated transcriptional enhancement of
Additional file 9:AHLgenes inArabidopsis thaliana.(a)Topology of the exon and intron arrangement ofArabidopsis thaliana AHLgenes.(b)The intron-mediated enhancement scores of the first/second/third/fourth intron were shown. The grey line represents a score of 10, which indicates moderate capability of transcriptional enhancement.(c)The intron-mediated enhancement scores of each intron inAtAHLs were listed. The abilities of transcriptional enhancement were categorized with strong (orange color), relatively strong (light orange), moderate (light blue) and weak (no color). (PDF 97 KB)
Expression analysis of the
Additional file 10:AHLgenes inArabidopsis thalianausing Genevestigator V3.0.年代imilarities between expression profiles ofAHLgenes were calculated using Manhattan Distance method (www.genevestigator.com) [67]. Type-IAHLs were labeled in blue color. Type-IIAHLs were labeled in green color. Type-IIIAHLs were labeled in red color. (PDF 590 KB)
Expression Analysis of the
Additional file 11:AHLGenes inZea maysUsing Genevestigator V3.0.年代imilarities between expression profiles ofAHLgenes were calculated using Pearson Correlation method (www.genevestigator.com) [67]. Type-IAHLs were labeled in blue color. Type-IIAHLs were labeled in green color. Type-IIIAHLs were labeled in red color. (PDF 349 KB)
Inferred orthologs and paralogs of the Clade-A
Additional file 12:AHLgenes in land plant species.(XLSX 228 KB)
推断直接同源和支系b的假字
Additional file 13:AHLgenes in land plant species.(XLSX 150 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
这篇文章是发表在生物医学的许可证Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, J., Favero, D.S., Qiu, J.et al.Insights into the evolution and diversification of theAT-hook Motif Nuclear Localizedgene family in land plants.BMC Plant Biol14,266 (2014). https://doi.org/10.1186/s12870-014-0266-7
Received:
Accepted:
Published:
Keywords
- AT-hook motif
- AT-Hook Motif Nuclear Localized (AHL) genes
- Diversification
- PPC domain
- Phylogeny