- Research article
- Open Access
- Published:
HSI2/VAL1 PHD-like domain promotes H3K27 trimethylation to repress the expression of seed maturation genes and complex transgenes in Arabidopsis seedlings
BMC Plant Biologyvolume14, Article number:293(2014)
Abstract
Background
The novel mutant allelehsi2-4was isolated in a genetic screen to identify Arabidopsis mutants with constitutively elevated expression of aglutathione S-transferase F8::luciferase(GSTF8::LUC) reporter gene in Arabidopsis. Thehsi2-4mutant harbors a point mutation that affects the plant homeodomain (PHD)-like domain in HIGH-LEVEL EXPRESSION OF SUGAR-INDUCIBLE GENE2 (HSI2)/VIVIPAROUS1/ABI3-LIKE1 (VAL1). Inhsi2-4seedlings, expression of thisLUCtransgene and certain endogenous seed-maturation genes is constitutively enhanced. The parental reporter line (WTLUC) that was used for mutagenesis harbors two independent transgene loci,KanRandKanS. Both loci express luciferase whereas only theKanRlocus confers resistance to kanamycin.
Results
Here we show that both transgene loci harbor multiple tandem insertions at single sites. Luciferase expression from these sites is regulated by the HSI2 PHD-like domain, which is required for the deposition of repressive histone methylation marks (H3K27me3) at bothKanRandKanSloci. Expression ofLUCandNeomycin Phosphotransferase IItransgenes is associated with dynamic changes in H3K27me3 levels, and the activation marks H3K4me3 and H3K36me3 but does not appear to involve repressive H3K9me2 marks, DNA methylation or histone deacetylation. However,hsi2-2andhsi2-4mutants are partially resistant to growth inhibition associated with exposure to the DNA methylation inhibitor 5-aza-2′-deoxycytidine. HSI2 is also required for the repression of a subset of regulatory and structural seed maturation genes in vegetative tissues and H3K27me3 marks associated with most of these genes are also HSI2-dependent.
Conclusions
These data implicate HSI2 PHD-like domain in the regulation of gene expression involving histone modifications and DNA methylation-mediated epigenetic mechanisms.
Background
Transition from seed maturation to seed germination and seedling development involves a complex network of genetic and epigenetic mechanisms that down-regulate the expression of seed maturation genes in seedlings [1] [6].Seed maturation is under the control of a group of transcriptional activators including LEAFY COTYLEDON1 (LEC1 [7]), LEC1-LIKE (L1L [8]), ABSCISIC ACID INSENSITIVE3 (ABI3 [9]), FUSCA3 (FUS3 [10]) and LEC2 [11], which are collectively called the “LAFL network” [3].包含转录repr的B3-domainsors HIGH-LEVEL EXPRESSION OF SUGAR-INDUCIBLE GENE2 (HSI2) /VP1/ABI3-LIKE1 (VAL1) and its homolog HSI2-LIKE1 (HSL1)/VAL2 act redundantly to repress ectopic activation of embryonic traits during seed germination and seedling development by the “LAFL network” of transcriptional activators [12] [16].HSI2 was also shown to negatively regulate the expression of β-glucuronidase (GUS) or luciferase (LUC) reporters under the control of seed-maturation specific gene promoters in transgenic Arabidopsis seedlings and vegetative organs [17],[18].Since many of the genes repressed by HSI2 in vegetative tissues are involved in the maturation phase of seed development, including desiccation tolerance, knock-outhsi2mutant seedlings show enhanced tolerance to water deficit whereas the overexpression ofHSI2resulted in hypersensitivity to desiccation stress [19].Recently, it was shown that bothfus3andlec2loss of function mutants can completely suppress the embryonic phenotype ofhsi2/hsl1double mutant seedlings, while it is partially suppressed inabi3,lec1andl1lmutants [15].These results indicate that HSI2 and HSL1 function redundantly to repress the expression of these regulatory genes in seedlings to prevent ectopic expression of embryonic traits during seed germination and vegetative development.
Developmental regulation of gene expression in plants is affected by chromatin mediated epigenetic mechanisms that include DNA methylation, chromatin remodeling, histone variants, and histone modifications [20],[21].DNA methylation at the 5′position of cytosine plays important roles in transcriptional silencing of transposons, repeat sequences, transgenes and transcribed genes [22].In addition to DNA methylation, histone modifications also play a vital role in the regulation of both transposons and transcribed genes in plants. Methylation of various lysine residues in the N-terminal tail of histone H3 is a well characterized epigenetic mechanism. In Arabidopsis, mono- (me1), di- (me2) or tri- (me3) methylation of histone H3 occurs mainly at lysine 4 (K4), lysine 9 (K9), lysine 27 (K27) and lysine 36 (K36) [23].H3K4me3 and H3K36me3 are enriched on actively transcribed genes whereas H3K27me3 marks are associated with developmental repression of transcribed genes. H3K9me2/3 marks, which are associated with DNA methylation and small interfering RNAs (siRNAs), are enriched in heterochromatic regions known to be involved in transcriptional silencing of transposons, repeat sequences and transgenes [23],[24].
HSI2 and HSL1 proteins were predicted to contain a PHD-like domain, a B3-DNA binding domain, a conserved cysteine and tryptophan residue-containing (CW) domain and an ethylene-responsive element binding factor-associated amphiphilic repression (EAR) motif [3],[12],[14],[17],[25],[26].Both CW and PHD protein domains are known to recognize methylated histone marks [23],[27] [30].Hoppmann et al. [29] showed that the CW domain of HSI2 binds to H3K4me2 and H3K4me3in vitroand, recently, it was reported that the HSL1 CW domain interacts with the histone deacetylase HDA19 to repress the “LAFL network” genes, includingLEC1andLEC2, by promoting histone deacetylation and the addition of H3K27me3 marks [31].However, molecular and epigenetic mechanisms underlying the HSI2 PHD-like domain-mediated regulation of gene expression remain to be elucidated.
Previously, we reported a novelHSI2allele,hsi2-4, in Arabidopsis that harbors a point mutation resulting in an amino acid substitution (C66Y) in the second zinc finger of the HSI2 PHD-like domain. Thehsi2-4mutant seedlings that carry aglutathione S-transferase F8::luciferase(GSTF8::LUC) reporter gene showed constitutively elevated transgene expression [14].In addition to theLUCtransgene, HSI2 PHD-like domain is required for the non-redundant repression of several seed-maturation genes in seedlings. These genes include those that encode both regulatory factors such as FUS3, and AGAMOUS-Like 15 (AGL15) and structural proteins that include cupin family storage protein, oleosins, late-embryogenesis-related proteins and seed storage albumins. Moreover, seed-specific genes that are de-repressed inhsi2-4mutant seedlings are targets of H3K27me3 marks. Chromatin immunoprecipitation and quantitative PCR (ChIP-qPCR) analyses indicated that HSI2 PHD-like domain promotes H3K27me3 marks on transgeneGSTF8promoter andLUCcoding sequences to repress transgene expression in parentalGSTF8::LUCreporter (WTLUC) seedlings [14].Both WTLUCandhsi2-4LUCmutant plants harbor two independent transgene loci [14].One locus, located on chromosome IV, confers kanamycin resistance and luminescence, whereas the second locus, which is on chromosome V, confers only luminescence. Based on kanamycin sensitivity, the chromosome IV and chromosome V loci were named asKanRandKanS, respectively [14].
In this work, we show that HSI2 PHD-like domain repressesLUCtransgene expression from bothKanRandKanSloci by promoting H3K27me3 marks but not DNA methylation and siRNA associated H3K9me2 marks. Expression ofNeomycin Phosphotransferase II (NPTII)from theKanRlocus is also partially suppressed in an HSI2-dependent mechanism. However, while our data indicate that DNA methylation and histone deacetylation are not involved in the transcriptional repression of transgene loci in WTLUC, the HSI2 PHD-like domain may play a role in the inhibition of seedling growth and development caused by DNA methylation inhibitor 5-aza-2′-deoxycytidine (5-azadC).
Results
Disruption of HSIPHD-like domain affects the expression of both KanRand KanStransgene loci
TheGSTF8::LUCreporter construct contains aGSTF8promoter sequence that controls the transcription of aluciferase表达ssion cassette, along with anNPTIIgene under control of thenopaline synthasepromoter and terminator sequences, which confers kanamycin resistance in plants (Figure1A). The parental WTLUCreporter line harbors two independent transgene insertion sites,KanRandKanS. TheKanRlocus was mapped to chromosome IV, while theKanSlocus is located on chromosome V (Table1) [14].Active luciferase is expressed by bothKanRandKanSloci, conferring a luminescent phenotype; however, only theKanRlocus expressesNPTII; thus, plants that harbor only theKanRlocus are resistant to kanamycin, whileKanSplants are sensitive to this antibiotic.
Genomic structure ofGSTF8::LUCtransgene and luminescence imaging of WTLUCandhsi2-4mutant seedlings harboring eitherKanRorKanS转基因locus or both. A.GSTF8::LUCtransgene containneomycin phosphotransferase(NPTII) coding sequences under the control ofnopaline synthase(NOS) promoter and a modifiedluciferase(LUC+)coding sequences from firefly driven byglutathione S-transferase F8(GSTF8) promoter conferring kanamycin resistance and luminescence expression respectively in plants. The 3′ ends of bothNPTIIandLUC+coding sequences includeNOSterminator sequences for transcriptional termination.B. Plants harboring eitherKanRorKanS转基因独自在野生型或轨迹hsi2-4mutant background were obtained by crossing of either WTLUCorhsi2-4LUCinto Columbia-0 wild-type and homozygous lines were identified in F2and F3generations. Five days old seedlings of various genotypes grown on Murashige and Skoog media plates were imaged using cooled CCD camera after spraying with the substrate luciferin. Pseudocolor image indicates luminescence intensity from lowest (blue) to highest (white).
To estimate the number ofLUCcopies at bothKanRandKanSloci, real-time quantitative PCR (qPCR) was performed using genomic DNA from WTLUC,KanRandKanSplants. Since bothKanRandKanSloci confer luminescence expression, we used PCR primers that are specific to theLUCcoding sequences to estimate the copy numbers. The results show thatKanSplants contain 2 copies ofLUCwhereas theKanRlocus harbors 5LUCcopies. Independent analysis of WTLUCplants, which contain bothKanRandKanSloci, showed seven copies of theLUCtransgene (Table1). Therefore, bothKanRandKanSloci are complex and contain multiple copies of theGSTF8::LUCtransgene.
Previously, we showed that disruption of the HSI2 PHD-like domain affects the expression of theKanR转基因locus [14] but the effect of this mutation on theKanSlocus was not evaluated. Therefore, to further investigate whether theKanS转基因locus is also regulated by the HSI2 PHD-like domain mutation and investigate potential interactions betweenKanRandKanS转基因loci in WTLUCandhsi2-4LUCmutant plants, these two loci were separated by crossing plants of the WTLUCreporter line and thehsi2-4LUCmutant line into Col-0 wild-type Arabidopsis and subsequent selection for homozygous WTLUCandhsi2-4lines that carry either theKanRorKanSreporter gene locus.
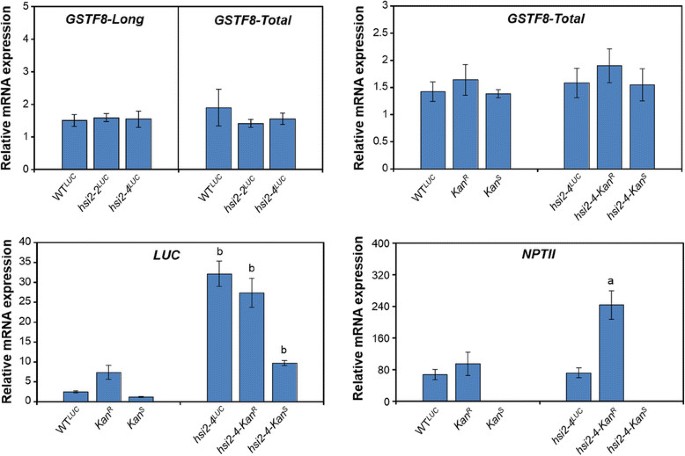
Comparison of luciferase expression in seedlings homozygous for the isolatedKanRandKanS转基因loci in the wild-type background showed thatKanRseedlings had higher luminescence signals (Figure1B) and steady state levels ofLUCmRNA (Figure2) thanKanSseedlings. This is in agreement with the relative number of transgene copies at these loci. However, in spite of carrying moreluciferasetransgene copies thanKanRseedlings, WTLUCseedlings, showed significantly lower luminescence signal andLUCtranscript levels. On the other hand, analysis of the expression of these transgenes in thehsi2-4background showed strongly enhancedluciferase表达ssion in all of the lines and the relative levels of both luminescence signal andLUCtranscripts corresponded with transgene copy number, with highest levels seen inhsi2-4LUCseedlings and lowest levels inhsi2-4-KanSsamples (Figures1B and2). This could indicate that, in a wild-type background, the presence of both theKanRandKansloci may lead to stronger suppression of transgene expression but disruption of the HSI2 PHD-like domain affects the expression ofLUCtransgenes at bothKanRandKanSloci similarly. Thus, the more complete HSI2-mediated repression of theGSTF8::LUCtransgenes in WTLUCplants results in stronger relative activation of their expression in the presence of thehsi2-4mutation.
Transcript levels of endogenousGSTF8and transgenes in WTLUCandhsi2mutants carrying eitherKanRorKanS转基因locus or both.Real-time reverse transcription quantitative PCR was used to determine the relative transcript levels of endogenousGSTF8,LUCandNPTIIgenes in five day old seedlings of various genotypes.GSTF8produces two different transcripts with different fragment lengths by alternative start sites namelyGSTF8-LongandGSTF8-Short[34].Expression ofGSTF8-Totalrepresents transcripts from bothGSTF8-LongandGSTF8-Shortversions whereasGSTF8-Long表达ssion level corresponds toGSTF8-Long成绩单。EF1αwas used for normalization. Data represent means (’sD) of two biological replicates with three technical replicates each. Significant differences inLUCtranscript levels between the three luciferase reporter lines in the wild-type background and the respectivehsi2-4mutant background, determined using two-tailed Student’st-test assuming unequal variances, are indicated by letters (a =p< 0.001 and b =p< 0.0001).
Alternative transcriptional start sites of the endogenousGSTF8结果在两个不同的transcrip基因ts with different sizes:GSTF8-Long(GSTF8-L) andGSTF8-Short(GSTF8-S) [34].To determine whether endogenousGSTF8表达ssion is altered inhsi2mutant alleles, we performed qRT-PCR using various wild type lines (WTLUC,KanRandKanS) andhsi2mutant lines (hsi2-2LUC,hsi2-4-KanR,hsi2-4-KanSandhsi2-4LUC).hsi2-2is a loss-of-function mutant allele that carries a T-DNA insertion in the seventh exon ofHSI2gene (SALK_088606) [12] [14],[17].To obtain thehsi2-2LUCline,GSTF8::LUCtransgenes were introgressed into thehsi2-2mutant background by genetic crossing. Expression ofGSTF8-Total(GSTF8-T) represents bothGSTF8-LandGSTF8-Stranscripts whereasGSTF8-Long表达ssion representsGSTF8-Ltranscripts only. As shown in Figure2, levels of endogenousGSTF8-LandGSTF8-Ttranscripts were not significantly affected inhsi2-2LUCandhsi2-4LUCplants andNPTII表达ssion was not detected in theKanSreporter line, consistent with the kanamycin sensitivity of these plants.NPTIItranscripts were expressed at similar levels in the WTLUCandhsi2-4LUCseedlings but expression ofNPTIIinKanRseedlings was responsive to the HSI2 PHD-like domain point mutation (Figure2). Steady-state levels ofNPTIItranscripts from theKanRlocus were about 3-fold higher inhsi2-4seedlings than in the wild-type background. Taken together, these results indicate that both theGSTF8::LUCandNOS::NPTIItransgenes of the T-DNA cassette are partially suppressed by HSI2 inKanRseedlings; however theNPTIIgenes at theKanSlocus may be fully silenced and/or contain loss-of-function mutations. Furthermore, in WTLUCseedlings where theKanSlocus is present, expression of bothLUCand theNPTIIgenes at theKanRis more strongly suppressed.
Luciferase expression is not affected by DNA methylation or histone deacetylation inhibitors
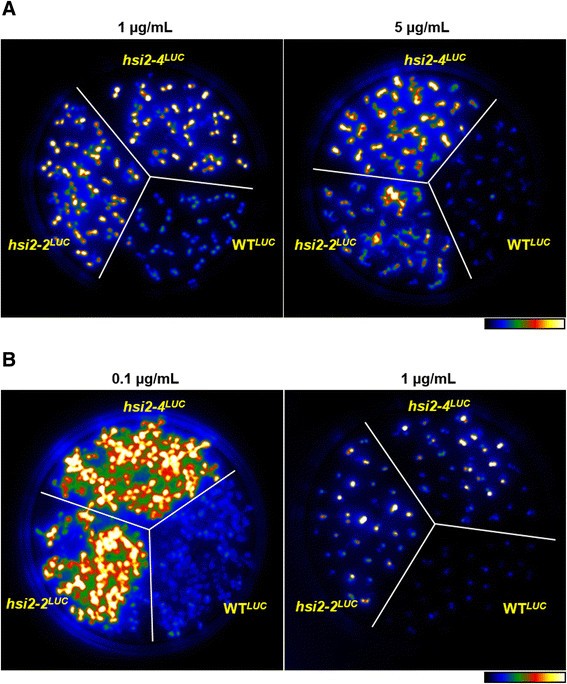
Complex transgenes with tandem repeats in plants are often subjected to DNA methylation and histone deacetylation mediated transcriptional gene silencing [35].If theGSTF8::LUC转基因loci in the WTLUCplants are targets of DNA methylation, treatment of these seedlings with an inhibitor of DNA methylation should derepress the luminescence expression similar to that seen inhsi2-4seedlings. Previous reports showed that treatment with 5 μM/mL of the DNA methylation inhibitor 5-aza-2′-deoxycytidine (5-azadC) was effective in derepressing the transcriptional silencing of auxin-responsive ß-GUS reporter lines [35].Treatment of Arabidopsis seedlings with 7 μM/mL 5-azadC also caused global changes in gene expression and derepression of silenced transgenes [36],[37].To investigate whether DNA methylation is involved in repressingGSTF8::LUCtransgene expression, WTLUC,hsi2-2LUCandhsi2-4LUCseedlings were grown on media containing 5-azadC at various concentrations. Luminescence expression in WTLUCseedlings was not affected at either 1 or 5 μM/mL 5-azadC concentrations (Figure3A). Hence, DNA-methylation does not appear to be required for the repression ofLUC表达ssion in WTLUCseedlings.
Treatments of WTLUC,hsi2-2LUCandhsi2-4LUCseedlings with DNA methylation inhibitor 5-aza-2′-deoxycytidine (5-azadC) and histone deacteylase inhibitor Trichostatin A (TSA).Seeds were germinated and grown vertically on media plates containing the indicated concentrations of either 5-azadC(A)or TSA(B). Luminescence imaging of 10 days old seedlings was performed using cooled CCD camera after spraying with the substrate luciferin. Pseudocolor images show luminescence intensity from lowest (blue) to highest (white). The experiment was repeated with two technical replicates and representative images are shown.
Histone deacetylation is known to regulate gene expression by transcriptional repression in eukaryotes [38].Recently, it was reported that treatment of Arabidopsis seedlings with the histone deacetylase inhibitor trichostatin A (TSA) or down-regulation of two histone deacetylase genes,HDA6andHDA19by RNA interference resulted in derepression of the embryonic program in germinating seeds and seedlings [39].Arabidopsis seedlings treated with TSA and histone deacetylase mutants mimic the phenotypes ofhsi2-2/hsl1double mutant seedlings [12],[13], indicating that HSI2- and HSL1-mediated repression of the embryonic program could involve histone deacetylation. HSL1 was shown to physically interact with HDA19 via its CW domain and disruption of HSL1 resulted in increased H3K4me3 and decreased H3K27me3 marks on genes that encode transcriptional activators involved in the embryonic program [31].To test the effects of TSA on the luminescence expression of WTLUCseedlings, WTLUC,hsi2-2LUCandhsi2-4LUCseedlings were grown on media containing 0.1 and 1 μg/mL TSA. Since higher concentrations of TSA resulted in severe growth retardation and developmental delay in all seedlings tested (Figure3B), only, 0.1 and 1 μg/mL of TSA was used in these assays. Luminescence imaging data showed that treatments of WTLUCseedlings with TSA did not affect their luminescence expression (Figure3B), indicating that HSI2 PHD-like domain mediated repression ofLUCtransgene expression in WTLUCseedlings is not dependent on TSA-sensitive histone deacetylation.
hsi2-2LUCand hsi2-4LUCmutant seedlings are partially resistant to DNA methylation inhibitor 5-azadC induced growth inhibition
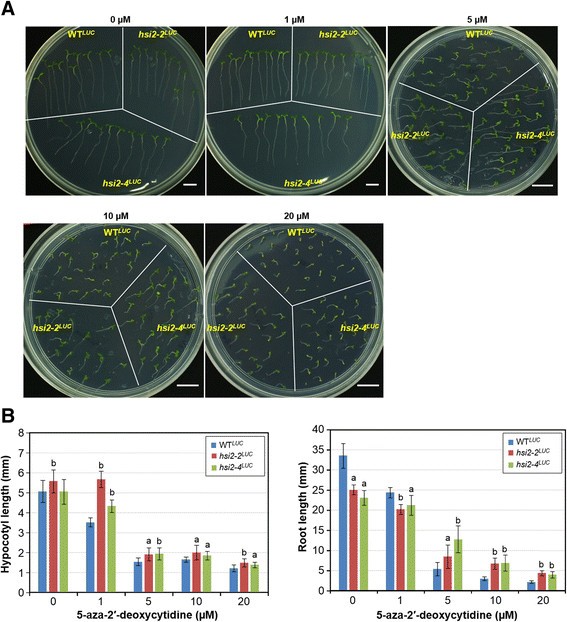
We noticed that the growth and development of WTLUCseedlings on plates that contained 5 μM/mL 5-azadC was more strongly inhibited thanhsi2-2LUCandhsi2-4LUCmutant seedlings (Figure4A). To further characterize the effects of 5-azadC on hypocotyl and root growth, WTLUC,hsi2-2LUCandhsi2-4LUCseeds were germinated on media containing 0, 1, 5, 10 and 20 μM 5-azadC. After 7 days of incubation on 5-azadC-containing media, all seedlings showed dose-dependent inhibition of growth and development. However, the most severe effects were seen with WTLUCseedlings whereas the growth ofhsi2-2LUCandhsi2-4LUCmutant seedlings was less inhibited (Figure4A). While WTLUCseeds germinated when incubated on media containing 20 μM 5-azadC, subsequent root growth and cotyledon development was almost completely abrogated while both andhsi2-4LUCandhsi2-2LUCmutant seedlings continued to grow and develop, albeit slowly, under these conditions (Figure4A and B). Comparative measurements of hypocotyl and root growth indicated thathsi2-2LUCandhsi2-4LUCmutant seedlings were about one half as sensitive to 5-azadC-dependent inhibition as WTLUCseedlings at 5, 10 and 20 μM 5-azadC treatments (Figure4B). These data indicate that, although 5-azadC does not affect the HSI2-dependent suppression of luciferase expression in WTLUCplants, HSI2 does affect sensitivity to 5-azadC-dependent inhibition of seedling development.
Effects of DNA methylation inhibitor 5-aza-2′-deoxycytidine (5-azadC) on the growth and development of WTLUC,hsi2-2LUCandhsi2-4LUCseedlings.Seeds were germinated vertically on media plates containing various concentrations of 5-azadC and pictures were taken 7 days after germination.A. Morphology of seedlings.B. Measurements of hypocotyl and root growths. Hypocotyl and root lengths were measured using ImageJ software. Data represent mean values (’sD) from 10 seedlings. The experiments were repeated with two technical replicates. Letters indicate significant differences between WTLUCandhsi2-2LUCorhsi2-4LUCat each time point (a =p< 0.005, b =p< 0.0001).
LUCandNPTIItransgene expression is associated with changes in histone methylation marks
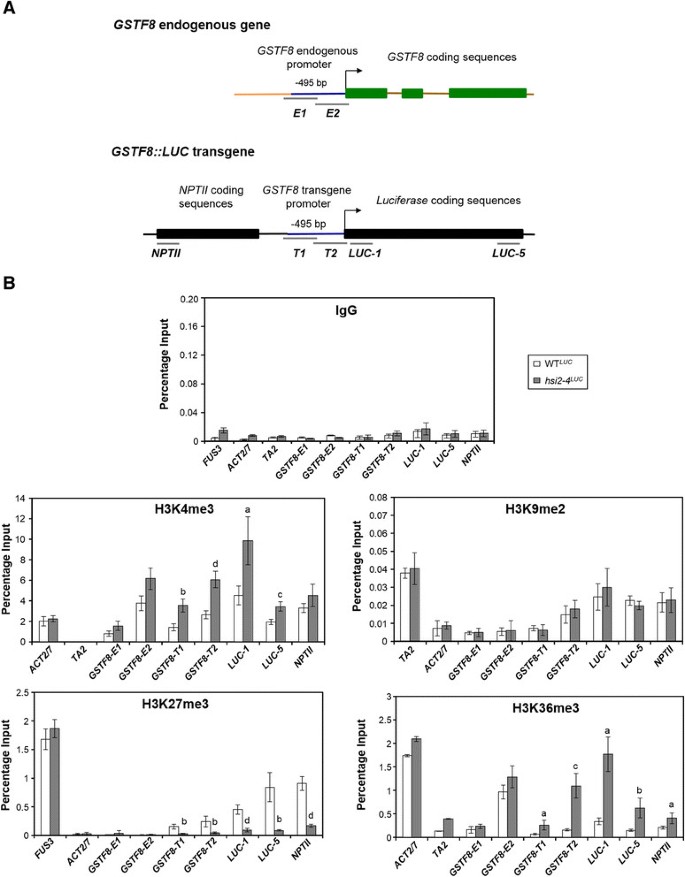
To examine the histone methylation properties along the transgene cassette and the role of HSI2 PHD-like domain in regulating those marks and transgene expression, ChIP-qPCR analyses were performed using 5 day old seedlings of various genotypes. Antibodies specific to H3K4me3, H3K9me2, H3K27me3 and H3K36me3 marks were used, along with PCR primers that specifically amplify sequences from the endogenous (native) and transgeneGSTF8promoters andLUCandNPTIIcoding regions. For the specific amplifications ofE1andE2PCR fragments only from the endogenousGSTF8promoter sequence during ChIP-qPCR, at least one PCR primer that binds outside of the −495 bp endogenousGSTF8region that is not part of theGSTF8::LUCtransgene cassette was used. Also, to make sure the PCR products ofT1andT2fragments are only amplified from theGSTF8transgene promoter sequence, at least one primer that binds outside of the −495 bp region in theGSTF8::LUCtransgene cassette was used ([14], Figure5A). PCR amplification specificities ofE1,E2,T1andT2fragments were confirmed using Col-0 wild-type and WTLUC. Among the histone methylation marks, H3K4me3 and H3K36me3 are associated with actively transcribed genes, while H3K9me2 is a repressive mark commonly enriched on transposable elements and repetitive sequences [24].H3K27me3 is a repressive mark associated with transcribed genes that are under tissue-specific or developmental regulation [40] [42].Preimmune immunoglobulin G (IgG) was used as a negative control for non-specific binding and all genomic DNA fragments tested show very low background levels of enrichment when chromatin samples were immunoprecipitated with IgG (Figure5B).FUS3was used as a positive control for H3K27me3, while actin2/7 (ACT2/7) was used as a negative control for H3K27me3 and as a positive control for H3K4me3 and H3K36me3.TA2was used as a positive control for H3K9me2 and as a negative control for H3K4me3 and H3K36me3. In agreement with our previous report [14], chromatin from the transgeneGSTF8promoter region, and both 5′and 3′ ends of theLUCcoding sequences was highly enriched in H3K27me3 marks in WTLUCseedlings (Figure5B) while endogenousGSTF8promoter sequences showed consistently low levels of H3K27me3 (Figure5B). Chromatin from the 5′region of theNPTIIcoding sequence was also highly H3K27me3 enriched in WTLUCseedlings (Figure5B). A substantial decrease in H3K27me3 levels was detected on chromatin from the transgeneGSTF8promoter sequences andLUCandNPTIIcoding sequences inhsi2-4LUCseedlings that carry a point mutation in HSI2 PHD-like domain (Figure5B). Though the transgene sequences tested showed considerable H3K9 dimethylation, unlike H3K27me3, no significant differences in H3K9me2 enrichment were seen between chromatin from WTLUCandhsi2-4LUCseedlings at any of the sites tested (Figure5B). Therefore, among the histone methylation marks associated with transcriptional suppression, only H3K27me3 was dependent on the HSI2 PHD-like domain.
Chromatin immunoprecipitation (ChIP) and quantitative PCR (qPCR) analyses of H3K4me3, H3K9me2, H3K27me3 and H3K36me3 levels on endogenousGSTF8promoter and transgene chromatin in WTLUCandhsi2-4LUCmutant. A. Genomic structures of endogenousGSTF8gene andGSTF8::LUCtransgene showing the locations of amplified regions by ChIP-qPCR.B. qPCR analyses of chromatin samples from 5-day old seedlings of WTLUCandhsi2-4LUCthat were immunoprecipitated using either specific antibodies recognizing indicated histone methylation marks or IgG (non-specific binding control). Data is expressed as percentage of immunoprecipitated DNA relative to input DNA.ACT2/7(H3K4me3 and H3K36me3),FUS3(H3K27me3) andTA2(H3K9me2) serve as positive controls whereasTA2(H3K4me3 and H3K36me3) andACT2/7(H3K9me2 and H3K27me3) were used as negative controls. Data represent means (’sD) from two biological replicates with three qPCR replicates each. Significant differences in enrichment between WTLUCandhsi2-4LUCfor each genomic region tested were determined using two-tailed Student’st-test assuming unequal variances andPvalues are indicated by letters (a =p< 0.05, b =p< 0.005, c =p< 0.0005, d =p< 0.0001).
Histone methylation marks H3K4me3 and H3K36me3, which are associated with chromatin from actively transcribed genes, were enriched at all of the transgene sequences assayed inhsi2-4LUCseedlings, relative to WTLUC(Figure5B). These marks were particularly abundant at the proximal transgeneGSTF8promoter and 5′LUCcoding sequences but significant enrichment was also seen at the endogenousGSTF8promoter andNPTIIcoding sequence.
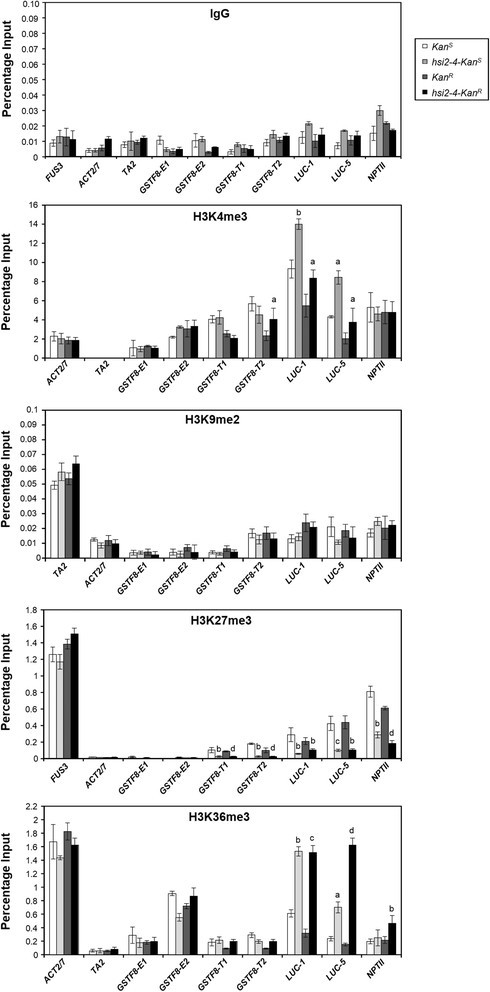
To examine whether thehsi2-4-dependent changes in histone methylation marks are associated with both theKanRandKanS转基因loci, ChIP-qPCR analyses were performed on various regions of the endogenousGSTF8gene and theGSTF8::LUCtransgene inKanR,KanS, hsi2-4-KanRandhsi2-4-KanSseedlings (Figure6). As in chromatin from WTLUCseedlings, higher levels of H3K27me3 marks at transgeneGSTF8promoter sequences and atLUCandNPTIIcoding sequences were detected in wild-type seedlings carrying either theKanRorKanS转基因locus than in correspondinghsi2-4-KanRorhsi2-4-KanSmutant seedlings. Thus, the significant decrease in H3K27me3 levels at theGSTF8::LUCtransgene associated with homozygosity for thehsi2-4allele was seen at both insertion sites. While chromatin from transgene sequences generally had higher levels of H3K9me2 marks than the endogenousGSTF8gene, no significant change was seen between these genotypes.
ChIP-qPCR analyses of H3K4me3, H3K9me2, H3K27me3 and H3K36me3 enrichments on endogenousGSTF8promoter andGSTF8::LUCtransgene regions in WTLUCandhsi2-4carrying eitherKanSorKanRlocus.染色质样本准备使用了5天seedlings from various genotypes. ChIP-qPCR data represents mean values (’sD) of three PCR reactions obtained from each of two independent immunoprecipitations. Significant differences between wild-type lines and corresponding mutant lines (KanSversushsi2-4-KanS,菅直人Rversushsi2-4-KanR) were determined using two-tailed Student’st-test assuming unequal variances andPvalues are indicated by letters (a =p< 0.05, b =p< 0.005, c =p< 0.0005, d =p< 0.0001).
Enrichment of H3K4me3 and H3K36me3 was seen in chromatin at bothKanRandKanSloci inhsi2-4seedlings (Figure6). This enrichment was most pronounced atLUCcoding sequences rather than in promoter regions and significant enrichment was also seen in chromatin of theNPTIIgene at theKanRlocus. Therefore, disruption of HSI2 PHD-like domain resulted in increased activation marks on 5′and 3′ end ofLUCcoding sequences in bothKanRandKanSbackgrounds. However, increased H3K36me3 marks on theNPTIIcoding sequences were observed only inhsi2-4-KanRseedlings (Figure6).
H3K27me3 levels are significantly decreased on a subset of seed-maturation genes in hsi2LUCmutant seedlings
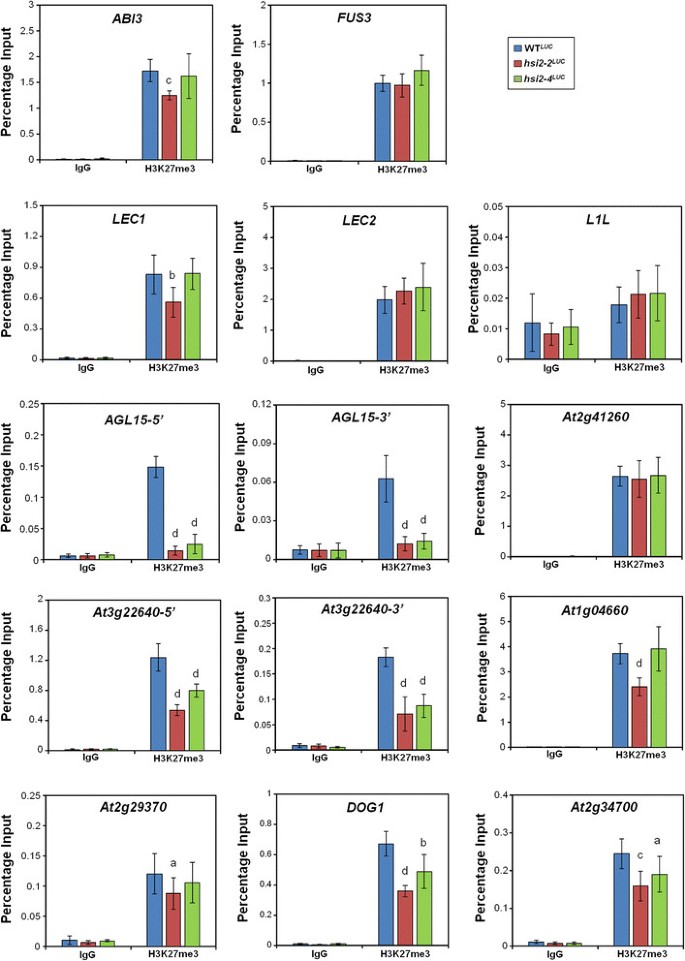
Some members of the LAFL clade of regulatory genes that control the expression of seed maturation genes [3] are misregulated inhsi2mutant seedlings [14].LEC1andABI3are ectopically expressed inhsi2-2but not inhsi2-4seedlings, whileFUS3is upregulated in bothhsi2-2andhsi2-4lines [14].These results suggested to us that the HSI2-dependent negative regulation ofLEC1andABI3in seedlings does not require the PHD-like domain, while suppression ofFUS3could be dependent on the PHD-like domain of HSI2. To determine if correlations exist between these expression patterns and histone modifications, ChIP-qPCR analysis of these genes was carried out on chromatin samples from WTLUC,hsi2-2LUCandhsi2-4LUCseedlings using antiH3K27me3 (Figure7). Consistent with our hypothesis, significant reductions in H3K27me3 chromatin marks were detected in association withABI3andLEC1genomic sequences only in chromatin fromhsi2-2LUCbut nothsi2-4LUCmutant seedlings and genes such asLEC2andL1L, which are not misregulated in eitherhsi2mutant allele alone, also showed no change in H3K27me3 marks in these mutant backgrounds. However, H3K27me3 marks associated withFUS3sequences were not altered in either mutant background. Thus, the effects of HSI2 onFUS3表达ssion do not appear to depend on alterations in H3K27me3. On the other hand, the enrichment of H3K27me3 marks detected on both 5′and 3′ coding sequences ofAGL15(At5g13790) in WTLUCseedlings was significantly decreased in bothhsi2-2LUCandhsi2-4LUCbackgrounds, which is consistent with the increased expression ofAGL15in these mutants [14].Therefore, the HSI2 PHD-like domain does appear to be required for both the repressed expression and H3K27 hypermethylation of theAGL15locus.
ChIP-qPCR analyses of H3K27me3 levels on the master transcriptional regulators of seed-maturation program and seed maturation related target genes of HSI2 PHD-like domain in WTLUCandhsi2LUCmutant seedlings.染色质样本准备使用了5天WTLUC,hsi2-2LUCandhsi2-4LUCseedlings. Immunoprecipitations were performed using either IgG (non-specific binding control) or anti-H3K27me3 antibody. Data represent means (’sD) obtained from three qPCR reactions each from three independent immunoprecipitations from three different biological replicates. Significant differences in H3K27me3 enrichments between WTLUCandhsi2LUCmutants (WTLUCversushsi2-2LUC, WTLUCversushsi2-4LUC) are indicated by letters (a =p< 0.05, b =p< 0.005, c =p< 0.0005, d =p< 0.0001).ABSCISIC ACID-INSENSITIVE3 (ABI3,At3g24650), FUSCA3 (FUS3,At3g26790), LEAFY COTYLEDON1 (LEC1,At1g21970), LEAFY COTYLEDON2 (LEC2,At1g28300), LEC1-LIKE (L1L,At5g47670), AGAMOUS-Like 15 (AGL15,At5g13790),At2g41260 (Late-embryogenesis-abundant protein), At3g22640 (cupin family seed storage protein), At1g04660 (glycine-rich protein), At2g29370 (NAD(P)-binding Rossmann-fold superfamily protein), At5g45830 (DOG1, DELAY OF GERMINATION1),At2g34700 (putative proline-rich glycoprotein).
As previously reported, a number of seed maturation-related structural genes are derepressed inhsi2-4mutant seedlings [14].To investigate whether the increased expression of these genes inhsi2mutant seedlings correlates with changes in associated H3K27me3 marks, ChIP-qPCR analyses were performed on chromatin from WTLUC,hsi2-2LUCandhsi2-4LUCmutant seedlings using primers specific to At2g41260 (Late-embryogenesis-abundant (LEA) protein), At3g22640 (cupin family seed storage protein), At1g04660 (glycine-rich protein), At2g29370 (NAD(P)-bindingRossmann-fold superfamily protein), At5g45830 (Delay of Germination1, DOG1) and At2g34700 (proline-rich glycoprotein). These seed maturation genes were previously reported to be targets of H3K27 hypermethylation [41] and our results confirmed that H3K27me3 marks were enriched, relative to IgG, on the 5′coding sequences of these genes (Figure7). In comparison to WTLUC,significant decreases in H3K27me3 enrichments were detected in bothhsi2-2LUCandhsi2-4LUCmutant seedlings on chromatin associated with 5′genomic sequences ofDOG1and At2g34700, and at both 5′and 3′ sequences of At3g22640 (Figure7). On the other hand, H3K27me3 levels on At1g04660 and At2g29370 were significantly decreased only in thehsi2-2LUCbackground and not in chromatin fromhsi2-4LUCseedlings (Figure7). These data indicate that, as with the regulatory genes described above, derepression of seed maturation-specific gene expression inhsi2突变体苗经常和减少对应accumulation of H3K27me3 marks that may or may not depend on the presence of an intact HSI2 PHD-like domain. However, as withFUS3, the LEA-like protein gene At2g41260, which is expressed at elevated levels in bothhsi2-2andhsi2-4mutant plants [14], is strongly enriched for H3K27me3 but these marks are not significantly reduced in eitherhsi2mutant.
Discussion
Despite detailed genetic and functional characterization, the molecular mechanisms that underlie HSI2- and HSL1-mediated repression of seed maturation program in seedlings are still not fully understood [12] [16].HSI2 contains a PHD-like domain [3],[12],[14],[26] and PHD domains can act as “readers” of the histone methylation status of target genes to regulate their expression [30].Through characterization of a novel mutant allele,hsi2-4, which affects the expression ofGSTF8::LUCtransgenes and certain seed maturation genes [14], we provide evidence that the HSI2 PHD-like domain is involved in regulating the expression of some genes by altering histone modifications.
Quantitative PCR data indicates that bothKanRandKanS转基因loci in the WTLUCreporter gene line contain multiple copies of theGSTF8::LUCtransgene. TheKanRlocus is more complex thanKanS, harboring five copies of the transgene, while theKanSlocus includes two (Table1). Transgene loci in plants that harbor multiple and complex transgene repeats at a single locus were frequently targeted by DNA methylation-associated H3K9me2 marks and also histone deacetylation mediated transcriptional gene silencing [35],[43] [45].However, treatment of WTLUCseedlings with either the DNA methylation inhibitor 5-azadC or the histone deacetylase inhibitor TSA failed to derepress theLUC表达ssion in WTLUCseedlings (Figure3A and B). Also, ChIP-qPCR analyses showed no differences in DNA methylation-associated H3K9me2 histone methylation marks on transgene sequences between WTLUCandhsi2-4mutants that harbor either individualKanRandKanSloci or both (Figures5B and6). Based on these data, it appears that DNA methylation and histone deacetylation mechanisms are not involved in HSI2 PHD-like domain-mediated repression of transgene expression in WTLUCseedlings.
Although 5-azadC did not affectLUC表达ssion in WTLUCseedlings (Figure3A),hsi2mutant seedlings treated with various concentrations of 5-azadC maintained root and hypocotyl growth better than WTLUCseedlings under the same conditions (Figure4A and B). These results could indicate that HSI2 is somehow involved in the inhibition of seedling growth and development caused by 5-azadC and, since this effect was observed with both the PHD-like domain mutant allelehsi2-4and thehsi2-2T-DNA knock-out allele, it appears that the PHD-like domain may be required for this 5-azadC-dependent inhibition of growth.LEC1, an HSI2 and HSL1 target gene and member of the “LAFL network”, was shown to be regulated by DNA methylation [46],[47] and the embryonic phenotypes of gain-of-functionlec1mutants were enhanced by treatment with a DNA methylation inhibitor 5-azacytidine [46].Hence, the partial rescue of seedling growth inhsi2mutants in the presence of 5-azadC could be an indirect effect of changes in the DNA methylation status of HSI2-targeted regulatory genes, includingLEC1.
Data presented here clearly indicate that the HSI2 PHD-like domain is involved in suppressing the expression ofGSTF8::LUCtransgenes in bothKanRandKanS转基因loci (Figure2). Furthermore, the levels ofLUC表达ssion seen inhsi2-4plants that carry these reporter complexes correlate with transgene copy number, with relatively low levels ofLUC表达ssion seen inhsi2-4,KanSplants that contain twoGSTF8::LUCcopies and correspondingly higher levels expression inhsi2-4, KanRorhsi2-4LUClines with five and seven total reporter gene copies, respectively. The direct correlation between derepressedLUC表达ssion and the number of reporter gene copies means that the luminescence of mutant plants with compromised HSI2-dependent repression will be amplified in a high copy number reporter gene line, making these mutants far more apparent in a luminescence-based mutant screen. On the other hand,LUC表达ssion in wild-type plants that carry theKanRlocus alone showed higher levels ofLUCtranscripts than did WTLUCseedlings (Figure2). These results appear to indicate that the presence of bothKanRandKanSloci in the same genome may lead to stronger transcriptional suppression of theGSTF8::LUCreporter genes than when theKanRlocus is present alone. NoNPTIItranscripts were detected in eitherKanSorhsi2-4-KanSseedlings (Figure2), which is consistent with the kanamycin sensitivity of these plants. However,hsi2-4-KanRseedlings showed 3-fold higher expression ofNPTIItranscripts relative to wild-typeKanRseedlings but this was not seen in correspondinghsi2-4LUCand WTLUCseedlings (Figure2). Thus, as with theLUCreporter gene, the co-existence ofKanRandKanSloci is associated with stronger transcriptional repression ofNPTIIgene expression. To better understand the function of the HSI2 PHD-like domain, interactions between activation-associated and repressive histone methylation marks at theKanRandKanS转基因loci were evaluated by ChIP-qPCR assays. TransgeneGSTF8promoter sequences, along withLUCandNPTII编码序列在H3K27me3高纯度marks in WTLUCseedlings (Figures5B and6) and significantly lower amounts of H3K27me3 were observed on these transgene sequences in thehsi2-4mutant background (Figure5B). Similar histone modification patterns were observed in seedlings harboring individualKanRorKanSloci (Figure6). Thus, the PHD-like domain of HSI2, which is required to repress the expression of these transgene complexes, is also necessary for the appearance of H3K27me3 marks on these loci. In contrast, H3K4me3 and H3K36me3 histone methylation marks, which are associated with active gene expression and have been shown to inhibit H3K27me3 marks on transcribed genes in both animals and plants [48] [50], were enriched on these transgene sequences inhsi2-4seedlings, relative to those with the wild-typeHSI2allele. Thus, the decrease in H3K27me3 marks on transgene sequences in bothKanRandKanSloci in thehsi2-4mutant background is associated with both increased expression (Figure2) and increased accumulation of H3K4me3 and H3K36me3 marks (Figures5B and6). Developmental repression of transcribed genes is often associated with H3K27me3 marks [41],[42] but emerging evidence also suggests that H3K27me3 may act as an alternative to DNA methylation-associated H3K9me2 in transposable elements and repetitive sequence silencing [40],[51] [54].Turck et al. [40] showed that the chromodomain-containing H3K27me3 “reader” protein LHP1 (LIKE HETEROCHROMATIN PROTEIN 1) is enriched on tandemly duplicated genes, such as the nine closely linked chitinase/glucosylase-18 genes (At4g19720-At4g19820) on chromosome 4 of Arabidopsis, but not on segmentally duplicated genes. Expressed genes that flank tandemly duplicated gene loci are also not associated with LHP1. BothKanRandKanSloci contain multipleGSTF8::LUCtransgenes at individual loci (Table1) and expression of genes that flank theKanR转基因locus does not differ between WTLUCandhsi2-4LUCseedlings [14].However, similarities inHSI2-dependent H3K27me3 accumulation at theKanRandKanSloci and the corresponding relative changes inLUC表达ssion inhsi2-4plants lead us to speculate that transcriptional repression is mediated by theGSTF8::LUCtransgene itself and is not dependent on tandem T-DNA insertions. The presence of multiple transgene copies results in high levels of expression that accentuates the apparent repressive effect of HSI2 when its activity is compromised by mutation. However, sinceLUC表达ssion in WTLUCseedlings is lower than inKanRseedlings, the presence of these two unlinked loci appears to have synergistic effects on reporter gene silencing.
In contrast to theGSTF8::LUCreporter genes, expression of nativeGSTF8记录来自“长”或“商店rt” transcriptional start sites show no significant increase in thehsi2-4LUCmutant background (Figure2). The most parsimonious explanation for the discrepancy between nativeGSTF8表达ssion and the expression of theGSTF8::LUCreporter gene is that the isolatedGSTF8promoter sequence used in theGSTF8::LUCgene construct, which corresponds with the short promoter as defined by Thatcher et al. [34], could containcis-acting suppressor elements that are masked in the context of the native gene. Support for this explanation can be seen in Figure4B of Veerappan et al. [14].In this experiment, LUC expression (measured as luminescence) was assayed in WT Col-0 and mutanthsi2-4Arabidopsis plants newly transformed with either short-GSTF8::LUCor long-GSTF8::LUCgene constructs (Col-S, Col-L andhsi2-4-S andhsi2-4-L, respectively). No significant differences in luminescence were apparent between Col-L andhsi2-4-L plants but LUC expression driven by the shortGSTF8promoter inhsi2-4-Splants was substantially elevated relative to that detected in Col-S plants.
Polycomb group (PcG) proteins are evolutionarily conserved multi-protein complexes required for developmental repression of gene expression by chromatin based mechanisms. PcG proteins in plants comprise of two major complexes: Polycomb Repressive Complex 1 (PRC1) and PRC2 [4] [6],[55].Arabidopsis PRC1 proteins BMI1 and RING1 were shown to have histone H2A mono ubiquitination (H2Aub) activityin vitro[16],[56],[57], whereas PRC2 complex proteins catalyze the deposition of H3K27me3 marks to promote developmental repression in animals and plants [58] [60].
TheGSTF8promoter sequence used in theGSTF8::LUCreporter contains anoctopine synthase(OCS)sequence element at −460 (Additional file1) that is known to be required for transcriptional activation in response to a variety of biotic and abiotic stress signals [61].ThisOCSelement is flanked byOCS element binding factor 5 (OBF5)andOCS element binding proteins 1 (OBP1)elements that were shown to bind proteins of theDNA binding with One Finger (DOF)family of transcription factors and are reported to act either as positive or negative regulatory factors in various plant genes [62].A putativemyeloblastosis2(MYB2) binding element is also located at −311 but its potential function is unknown. Sequence elements with a potential role in PRC2-based silencing can also be identified in theGSTF8启动子。通过分析co-distribution的ubiquitous Arabidopsis PRC2 protein FERTILIZATION INDEPENDENT ENDOSPERM (FIE) and H3K27me3 marks, Deng et al. [63] identified four sequence motifs that could act as PRC2 binding sites. Two of these motifs are found in theGSTF8子,一个假定的GAGA框位于−123之间and −131 relative to the transcription start site and a TTC repeat element located in the 5′untranslated region between +36 and +56. GAGA elements were found to be specifically associated with FIE and H3K27me3 enriched sites and these sequences are also found in polycomb response elements (PREs) of Drosophila [63].However, while GAA (reverse complement of TTC) sequence motifs were found to be associated with genomic regions that bind both FIE and H3K27me3, they were also enriched in random promoter sequences and were, therefore, not considered to be specific PRC2 binding sites [63].
The role of the GAGA element in the transcriptional regulation of the PRC2-repressedLEC2gene was confirmed by Berger et al. [64].However, mutational analysis showed that, in this context, the GAGA element acted as a requiredcis-activating element, which was associated with a distinctcis-repressing element, termed repressive LEC2 element (RLE) that apparently consists of two component sequences. Comparison of theLEC2RLE sequence with theGSTF8promoter identified a duplicated element identical to the 5′component of theLEC2RLE. As in theLEC2gene, the putativeGSTF8RLE-like sequence is located immediately downstream of the GAGA element (Additional file1). Whether this putative GAGA-RLE motif plays a role in the transcriptional regulation of theGSTF8promoter in a transgene context is not known.
ChIP-qPCR analyses were carried out to investigate whetherLAFLnetwork genes and other seed maturation genes that are up-regulated inhsi2mutant seedlings are also associated with HSI2-dependent changes in H3K27me3 marks. With the exception ofL1L, all the tested seed maturation genes are enriched with H3K27me3 marks in WTLUCseedlings (Figures7) in agreement with previous reports [41],[58].Significant reductions in H3K27me3 levels, relative to WTLUCseedlings, were observed at some gene loci in bothhsi2-2LUCandhsi2-4LUCseedlings, while other genes showed reductions only inhsi2-2LUCseedlings and a few showed no changes in eitherhsi2mutant background. In general, these HSI2-dependent differences in H3K27me3 marks correlate well with HSI2-dependent changes in gene expression. For example, among the regulatory genes tested,ABI3andLEC1, which are expressed at elevated levels inhsi2-2seedlings but not inhsi2-4seedlings [14], showed correspondingly decreased levels of H3K32me3 enrichment in chromatin fromhsi2-2LUCplants but not fromhsi2-4LUCplants (Figure7). On the other hand, H3K27me3 marks onAGL15were strongly decreased in bothhsi2-2LUCandhsi2-4LUCmutant plants (Figure7) and expression of this gene is also upregulated in bothhsi2mutant backgrounds. These results can be interpreted to indicate that HSI2-dependent transcriptional repression and H3K27 hypermethylation ofAGL15is mediated by the PHD-like domain while that ofABI3andLEC1may be mediated by other HSI2 domains, such as the CW domain, which was shown to interact with HDA19 to promote histone deacetylation and H3K27me3 marks to repress seed maturation genes [31].
Similar patterns can be seen in the structural (non-regulatory) seed maturation gene sample. The putative glycine-rich protein gene (At1g04660) is more strongly expressed inhsi2-2LUCplants than inhsi2-4LUCplants [14] and H3K27me3 marks at this site are correspondingly reduced inhsi2-2LUCbut nothsi2-4LUCmutants. On the other hand, genes for a cupin-like protein (At3g22640),DOG1(At5g45830) and a proline-rich glycoprotein (At2g34700) are similarly up-regulated inhsi2-2LUCandhsi2-4LUCplants and decreased H3K27me3 marks are also apparent in both mutant lines.
Two of the genes in our sample group do not show correlations between HSI2-dependent transcriptional repression and H3K27me3 marks. These genes,FUS3and the LEA protein gene At2g41260, are expressed at elevated levels in bothhsi2-2LUCandhsi2-4LUCbackgrounds and accumulate substantial H3K27me3 in WTLUCplants but these marks are not diminished in eitherhsi2mutant. Therefore, derepression of these genes inhsi2mutant plants does not appear to require the depletion of H3K27me3. It seems likely that, in addition to the PRC2-mediated accumulation of H3K27me3 marks, other mechanisms are involved in the repression of these genes.
AGL15 is a member of the MIKC subfamily of MADS domain transcription factors that is preferentially expressed in developing embryos. Ectopic overexpression ofAGL15结果掺anced somatic embryogenesis [65],[66].AGL15 acts upstream ofLAFLnetwork genes and severalLAFLgenes are direct regulatory targets of AGL15 [67],[68].DOG1is a seed-specific gene and plays a critical role in promoting seed dormancy by integrating environmental signals [69],[70].Recently, AGL15 and DOG1 were shown to be targets of H3K27me3 marks, which could be mediated by PRC1 proteins [58],[71].Our data shows that HSI2 regulates the expression ofAGL15andDOG1in seedlings by promoting H3K27me3 marks possibly via PRC1-PRC2 complex which requires HSI2 PHD-like domain.
HSI2 was shown to directly interact with PRC1 complex proteins AtBMI1A/B/C and is required for the deposition of H2Aubi and H3K27me3 marks on “LAFL network” seed-maturation genes includingLEC1,FUS3andABI3[16].Disruption of PRC2 complex genes in Arabidopsis led to decreased H3K27me3 levels, activation of “LAFL network” transcription factor genes and ectopic expression of embryonic traits during seed germination and vegetative development [58],[72],[73].Thus, it is possible that HSI2 interacts with PRC1 proteins like AtBMI1 to recruit PRC2 proteins such as the histone methyltransferase CURLY LEAF (CLF), to deposit H3K27me3 marks on theGSTF8::LUC转基因loci. AtRING1a was also shown to physically interact with the PRC2 core component CLF [74] and several reports have demonstrated the involvement of PHD-PRC2 and PHD-PRC1-PRC2 complexes in deposition of H3K27me3 marks to promote transcriptional repression of gene expression in plants [16],[71],[75].Similarly, HSI2 could be part of a repression complex that involves the HSI2 PHD-like domain, PRC1 and PRC2 complex proteins to promote high levels of H3K27me3 marks on native seed maturation genes andGSTF8::LUC转基因loci to repress their expression during the seed to seedling developmental phase transition.
Conclusions
HSI2 contains a putative PHD domain, which could act as a “reader” of histone methylation marks. In this work, we show that HSI2 PHD-like domain regulates bothLUCandNPTIItransgenes from two independent transgene loci. Transcriptional repression of both of these transgene loci by HSI2 PHD-like domain is associated with repressive histone methylation marks H3K27me3 but not siRNA and DNA methylation associated H3K9me2 marks. In addition to the transgenes, HSI2 is also required for the repression of a subset of seed maturation genes in seedlings by promoting H3K27me3 marks in a PHD-like domain dependent and independent manner.
Methods
Plant materials, growth conditions and chemical treatments
Arabidopsis thalianaColumbia-0 (Col-0; CS60000) wild-type was obtained from Arabidopsis Biological Resources Center. WTLUCandhsi2-4LUC, which contain bothKanRandKanS转基因loci in Col-0 background were described before [14].The other genotypes that were used in this study includingKanR,KanS,hsi2-4-KanRandhsi2-4-KanS通过穿越WT吗LUCandhsi2-4LUCinto the Col-0 wild-type. Thehsi2-2LUCline harborsGSTF8::LUCtransgenes in theHSI2T-DNA knock-out allelehsi2-2(SALK_088606) background. For all the experiments described here, plants were grown under continuous illumination at 24°C on 0.3% Phytagel plates containing 0.5X Murashige and Skoog (MS) salt, 0.5 g/L MES (2-(N-morpholino) ethanesulfonic acid, 1X Gamborg vitamin mix and 1 % sucrose (pH adjusted to 5.7). 5-Aza-2′-deoxycytidine (5-azadC, A3656; Sigma) and Trichostatin A (T8552; Sigma) stocks were prepared using dimethyl sulfoxide and methanol respectively, and added directly to the MS media plates. Hypocotyl and root length measurements were made using Image J software (http://rsbweb.nih.gov/ij/). Digital photos of seedlings grown on 5-azadC plates were taken along with a ruler of known length. Hypocotyl length was measured from the tip of the apical meristem to the junction between hypocotyl and root, while root length was measured from the hypocotyl/root junction to the tip of the primary root.
Luminescence imaging, genetic crosses and genotyping
Luminescence imaging was performed using Andor iKON-M DU934N-BV CCD camera (Andor Technology). After spraying with 1 mM D-luciferin potassium salt (Gold Biotechnology) containing 0.01% Triton X-100 solution, seedlings were kept in the dark for 5 minutes and imaging was performed with a 5 minute exposure. Andor SOLIS (I) imaging software (Andor Technology) was used for the acquisition of luminescence images and processing. To separateKanRandKanSloci, WTLUCandhsi2-4LUCmutant were crossed into Col-0 wild-type plants. Successful crosses were identified based on luciferase imaging in the F1generation and plants were allowed to self-pollinate. Progeny lines homozygous for theKanSlocus were identified based on kanamycin sensitivity whereas plants homozygous for theKanRlocus were identified by PCR genotyping using T-DNA and genomic primers. To genotypehsi2-4突变,先前描述的帽子标记(14] was used.
Preparation of total RNA, cDNA synthesis and real-time reverse transcription quantitative PCR
Total RNA extraction and real-time reverse transcription quantitative-PCR (RT-qPCR) analysis was performed as described in Veerappan et al. [14].Primers used in RT-qPCR are listed in Additional file2.
Estimation ofLUCtransgene copy numbers by real-time quantitative PCR
To determine the copy numbers ofLUCtransgenes in WTLUC,KanRandKanSlines, real-time quantitative PCR (qPCR) was performed as described before [32],[33].All qPCR reactions were performed using AB StepOnePlus Real-Time PCR System (Applied Biosystems) in 10 μl volume containing different amounts of DNA, 0.2 μM of each primers and 5 μl iTaqTM SYBR green supermix (Bio-Rad). Several sets of primers were tested for optimal performance. Temperature cycling conditions were 95°C for 10 minutes, 40 cycles for 15 seconds at 95°C and 1 minute at 60°C. Each DNA sample was tested in triplicates with three different DNA concentrations. Calibration curves were also performed in triplicates with five different DNA concentrations. Ct values were calculated using StepOne Software v2.1 (Applied Biosysytems). Concentrations of DNA samples were measured using Nanodrop 2000 (Thermoscientific) and the exact copy numbers of the template genome in the reactions were calculated using the following website:http://cels.uri.edu/gsc/cndna.html), applying the formula: number of copies = (amount * 6.022×1023)/(length * 1×109* 650). The calibration curves forLUCwere created using the plasmid DNA pBI121-GSTF8::LUCas a template. At5g47480, a single copy gene from Arabidopsis, was used as an internal control for normalization of the data. PCR primers used in the estimation of transgene copy numbers can be found in Additional file2.
Chromatin immunoprecipitation and quantitative PCR analyses
Chromatin immunoprecipitation (ChIP) and quantitative PCR (qPCR) analyses were performed as described by Veerappan et al. [14].Percentage of immunoprecipitated DNA relative to the total chromatin input was calculated for various samples using qPCR. Antibodies used for ChIP: normal rabbit IgG (Millipore, 12–370), anti-H3K4me3 (Millipore, 07–473), anti-H3K9me2 (Abcam, ab1220), anti-H3K27me3 (Millipore, 07–449) and anti-H3K36me3 (Abcam, ab9050). Primers used for ChIP PCR analyses are listed in Additional file2.
Authors’ contributions
VV designed and performed genetic crosses, luminescence imaging, qRT-PCR, DNA methylation and histone deacetylase inhibitor experiments, chromatin immunopreciptation and qPCR analyses. NC and AR designed and performed the estimation ofLUCcopy numbers in variousGSTF8::LUCtransgene reporter lines. VV and RDA coordinated all the experiments, wrote and edit the manuscript. All authors read and approved the final manuscript.
Additional files
Abbreviations
- HSI2:
-
High-level expression of sugar inducible gene2
- HSL1:
-
HSI2-Like1
- VAL1:
-
Viviparous1/ABI3-Like1
- PHD:
-
Plant homeodomain
- LUC:
-
Luciferase
- 5-azadC:
-
5-aza-2′-deoxycytidine
- GSTF8:
-
:LUC: GlutathioneS-transferase F8::luciferase
- NPTII:
-
Neomycin phosphotransferase II
- LEC1:
-
Leafy cotyledon1
- LEC2:
-
Leafy cotyledon2
- L1L:
-
LEC1-Like
- ABI3:
-
Abscisic acid insensitive3
- FUS3:
-
FUSCA3
- LAFL:
-
AFL (ABI3/ FUS3/ LEC2)/LEC (LEC1/L1L)
- GUS:
-
β-glucuronidase
- CW:
-
Cysteine and tryptophan residue-containing
- EAR:
-
Ethylene-responsive element binding factor-associated amphiphilic repression
- siRNAs:
-
Small interfering RNAs
- ChIP:
-
Chromatin immunoprecipitation
- qPCR:
-
Quantitative PCR
- WTLUC:
-
Wild-type luciferase
- KanR:
-
Kanamycin resistant
- KanS:
-
Kanamycin sensitive
- AGL15:
-
AGAMOUS-Like15
- GSTF8-L:
-
GSTF8-long
- GSTF8-S:
-
GSTF8-short
- TSA:
-
Trichostatin A
- ACT2/7:
-
Actin2/7
- IgG:
-
Immunoglobulin G
- H3K27me3:
-
Trimethylation of histone H3 at lysine 27
- H3K4me3:
-
Trimethylation of histone H3 at lysine 4
- H3K36me3:
-
Trimethylation of histone H3 at lysine 36
- H3K9me2:
-
Dimethylation of histone H3 at lysine 9
- LEA:
-
Late embryogenesis abundant
- DOG1:
-
Delay of germination1
- LHP1:
-
Like heterochromatin protein1
- PcG:
-
Polycomb group
- PRC1:
-
Polycomb repressive complex 2
- PRC2:
-
Polycomb repressive complex 1
- H2Aub:
-
H2A mono ubiquitination
- OCS:
-
Octopine synthase
- OBF5:
-
OCS element binding factor 5
- OBP1:
-
OCS element binding proteins 1
- DOF:
-
DNA binding with one finger
- FIE:
-
受精独立胚乳
- MYB2:
-
Myeloblastosis2
- PREs:
-
Polycomb response elements
- RLE:
-
Repressive LEC2 element
- CLF:
-
Curly leaf
References
Zhang H, Ogas J: An epigenetic perspective on developmental regulation of seed genes. Mol Plant. 2009, 2: 470-627. 10.1093/mp/ssp027.
Muller K, Bouyer D, Schnittger A, Kermode AR: Evolutionarily conserved histone methylation dynamics during seed life-cycle transitions. PLoS One. 2012, 7: e51532-10.1371/journal.pone.0051532.
Jia H, Suzuki M, McCarty DR: Regulation of the seed to seedling developmental phase transition by the LAFL and VAL transcription factor networks. Wires Dev Biol. 2014, 3: 135-145. 10.1002/wdev.126.
Calonje M: PRC1 marks the difference in plant PcG repression. Mol Plant. 2014, 7: 459-471. 10.1093/mp/sst150.
Derkacheva M, Hennig L: Variations on a theme: polycomb group proteins in plants. J Exp Bot. 2014, 65: 2769-2784. 10.1093/jxb/ert410.
Molitor A, Shen W-H: The polycomb complex PRC1: composition and function in plants. J Genet Genomics. 2013, 40: 231-238. 10.1016/j.jgg.2012.12.005.
Lotan T, Ohto M, Yee KM, West MA, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ: Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell. 1998, 93: 1195-1205. 10.1016/S0092-8674(00)81463-4.
Kwong RW, Bui AQ, Lee H, Kwong LW, Fischer RL, Goldberg RB, Harada JJ: LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development. Plant Cell. 2003, 15: 5-18. 10.1105/tpc.006973.
Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM: Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992, 4: 1251-1261. 10.1105/tpc.4.10.1251.
Luerssen H, Kirik V, Herrmann P, Misera S: FUSCA3 encodes a protein with a conserved VP1/AB13-like B3 domain which is of functional importance for the regulation of seed maturation inArabidopsis thaliana. Plant J. 1998, 15: 755-764. 10.1046/j.1365-313X.1998.00259.x.
Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ: LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci U S A. 2001, 98: 11806-11811. 10.1073/pnas.201413498.
Suzuki M, Wang HHY, McCarty DR: Repression of the LEAFY COTYLEDON 1/B3 regulatory network in plant embryo development by VP1/ABSCISIC ACID INSENSITIVE 3-LIKE B3 genes. Plant Physiol. 2007, 143: 902-911. 10.1104/pp.106.092320.
Tsukagoshi H, Morikami A, Nakamura K: Two B3 domain transcriptional repressors prevent sugar-inducible expression of seed maturation genes in Arabidopsis seedlings. Proc Natl Acad Sci U S A. 2007, 104: 2543-2547. 10.1073/pnas.0607940104.
Veerappan V, Wang J, Kang M, Lee J, Tang Y, Jha AK, Shi H, Palanivelu R, Allen RD: A novel HSI2 mutation in Arabidopsis affects the PHD-like domain and leads to derepression of seed-specific gene expression. Planta. 2012, 236: 1-17. 10.1007/s00425-012-1630-1.
Jia H, McCarty DR, Suzuki M: Distinct roles of LAFL network genes in promoting the embryonic seedling fate in the absence of VAL repression. Plant Physiol. 2013, 163: 1293-1305. 10.1104/pp.113.220988.
Yang C, Bratzel F, Hohmann N, Koch M, Turck F, Calonje M: VAL- and AtBMI1-mediated H2Aub initiate the switch from embryonic to postgerminative growth in Arabidopsis. Curr Biol. 2013, 23: 1324-1329. 10.1016/j.cub.2013.05.050.
Tsukagoshi H, Saijo T, Shibata D, Morikami A, Nakamura K: Analysis of a sugar response mutant of Arabidopsis identified a novel B3 domain protein that functions as an active transcriptional repressor. Plant Physiol. 2005, 138: 675-685. 10.1104/pp.104.057752.
Tang X, Hou A, Babu M, Nguyen V, Hurtado L, Lu Q, Reyes JC, Wang A, Keller WA, Harada JJ, Tsang EW, Cui Y: The Arabidopsis BRAHMA chromatin-remodeling ATPase is involved in repression of seed maturation genes in leaves. Plant Physiol. 2008, 147: 1143-1157. 10.1104/pp.108.121996.
Sharma N, Bender Y, Boyle K, Fobert PR:High-level expression of sugar inducible gene2 (HSI2)is a negative regulator of drought stress tolerance in Arabidopsis. BMC Plant Biol. 2013, 13: 170-10.1186/1471-2229-13-170.
Ahmad A, Zhang Y, Cao XF: Decoding the epigenetic language of plant development. Mol Plant. 2010, 3: 719-728. 10.1093/mp/ssq026.
Feng S, Jacobsen SE, Reik W: Epigenetic reprogramming in plant and animal development. Science. 2010, 330: 622-627. 10.1126/science.1190614.
Law JA, Jacobsen SE: Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010, 11: 204-220. 10.1038/nrg2719.
Liu C, Lu F, Cui X, Cao X: Histone methylation in higher plants. Annu Rev Plant Biol. 2010, 47: 395-420. 10.1146/annurev.arplant.043008.091939.
He G, Elling AA, Deng XW: The epigenome and plant development. Annu Rev Plant Biol. 2011, 62: 411-435. 10.1146/annurev-arplant-042110-103806.
Perry J, Zhao YD: The CW domain, a structural module shared amongst vertebrates, vertebrate-infecting parasites and higher plants. Trends Biochem Sci. 2003, 28: 576-580. 10.1016/j.tibs.2003.09.007.
Suzuki M, McCarty DR: Functional symmetry of the B3 network controlling seed development. Curr Opin Plant Biol. 2008, 11: 548-553. 10.1016/j.pbi.2008.06.015.
Lee WY, Lee D, Chung WI, Kwon CS: Arabidopsis ING and Alfin1-like protein families localize to the nucleus and bind to H3K4me3/2 via plant homeodomain fingers. Plant J. 2009, 58: 511-524. 10.1111/j.1365-313X.2009.03795.x.
He F, Umehara T, Saito K, Harada T, Watanabe S, Yabuki T, Kigawa T, Takahashi M, Kuwasako K, Tsuda K: Structural insight into the zinc finger CW domain as a histone modification reader. Structure. 2010, 18: 1127-1139. 10.1016/j.str.2010.06.012.
Hoppmann V, Thorstensen T, Kristiansen PE, Veiseth SV, Rahman MA, Finne K, Aalen RB, Aasland R: The CW domain, a new histone recognition module in chromatin proteins. EMBO J. 2011, 30: 1939-1952. 10.1038/emboj.2011.108.
Sanchez R, Zhou MM: The PHD finger: a versatile epigenome reader. Trends Biochem Sci. 2011, 36: 364-372.
Zhou Y, Tan B, Luo M, Li Y, Liu C, Chen C, Yu CW, Yang S, Dong S, Ruan J, Yuan L, Zhang Z, Zhao L, Li C, Chen H, Cui Y, Wu K, Huang S: HISTONE DEACETYLASE19 interacts with HSL1 and participates in the repression of seed maturation genes in Arabidopsis seedlings. Plant Cell. 2013, 25: 134-148. 10.1105/tpc.112.096313.
Mason G, Provero P, Varia AM, Acotto GP: Estimating the number of integrations in transformed plants by quantitative real-time PCR. BMC Biotechnol. 2003, 2: 20-10.1186/1472-6750-2-20.
Gadaleta A, Giancaspro A, Cardone MF, Blanco A: Real-time PCR for the detection of precise transgene copy number in durum wheat. Cell Mol Biol Lett. 2011, 16: 652-668. 10.2478/s11658-011-0029-5.
Thatcher LF, Carrie C, Andersson CR, Sivasithamparam K, Whelan J, Singh KB: Differential gene expression and subcellular targeting of Arabidopsis glutathioneS-transferase F8 is achieved through alternative transcription start sites. J Biol Chem. 2007, 282: 28915-28928. 10.1074/jbc.M702207200.
Murfett J, Wang XJ, Hagen G, Guilfoyle TJ: Identification of Arabidopsis histone deacetylase HDA6 mutants that affect transgene expression. Plant Cell. 2001, 13: 1047-1047-10.1105/tpc.13.5.1047.
Chang S, Pikaard CS: Transcript profiling in Arabidopsis reveals complex responses to global inhibition of DNA methylation and histone deacetylation. J Biol Chem. 2005, 280: 796-804. 10.1074/jbc.M409053200.
Dinh TT, O’Leary M, Won SY, Li S, Arroyo L, Liu X, Defries A, Zheng B, Cutler SR, Chen X: Generation of a luciferase-based reporter for CHH and CG DNA methylation in Arabidopsis thaliana. Silence. 2013, 4: 1-10.1186/1758-907X-4-1.
Hollender C, Liu Z: Histone deacetylase genes in Arabidopsis development. J Integrative Plant Biol. 2008, 50: 875-885. 10.1111/j.1744-7909.2008.00704.x.
Tanaka M, Kikuchi A, Kamada H: The Arabidopsis histone deacetylases HDA6 and HDA19 contribute to the repression of embryonic properties after germination. Plant Physiol. 2008, 146: 149-161. 10.1104/pp.107.111674.
Turck F, F Roudier, Farrona年代,Martin-Magniette毫升, Guillaume E, Buisine N, Gagnot S, Martienssen RA, Coupland G, Colot V: Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 2007, 3: e86-10.1371/journal.pgen.0030086.
Zhang X, Clarenz O, Cokus S, Bernatavichute YV, Pellegrini M, Goodrich J, Jacobsen SE: Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 2007, 5: e129-10.1371/journal.pbio.0050129.
Lafos M, Kroll P, Hohenstatt ML, Thorpe FL, Clarenz O, Schubert D: Dynamic regulation of H3K27 trimethylation during Arabidopsis differentiation. PLoS Genet. 2011, 7: e1002040-10.1371/journal.pgen.1002040.
Gong ZH, Morales-Ruiz T, Ariza RR, Roldan-Arjona T, David L, Zhu J-K: ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell. 2002, 111: 803-814. 10.1016/S0092-8674(02)01133-9.
To TK, Kim JM, Matsui A, Kurihara Y, Morosawa T, Ishida J, Tanaka M, Endo T, Kakutani T, Toyoda T, Kimura H, Yokoyama S, Shinozaki K, Seki M: Arabidopsis HDA6 regulates locus-directed heterochromatin silencing in cooperation with MET1. PLoS Genet. 2011, 7: e1002055-10.1371/journal.pgen.1002055.
Weinhold A, Kallenbach M, Baldwin IT: Progressive 35S promoter methylation increases rapidly during vegetative development in transgenicNicotiana attenuateplants. BMC Plant Biol. 2013, 13: 99-10.1186/1471-2229-13-99.
Casson SA, Lindsey K: The turnip mutant of Arabidopsis reveals that LEAFY COTYLEDON1 expression mediates the effects of auxin and sugars to promote embryonic cell identity. Plant Physiol. 2006, 142: 526-541. 10.1104/pp.106.080895.
Shibukawa T, Yazawa K, Kikuchi A, Kamada H: Possible involvement of DNA methylation on expression regulation of carrotLEC1gene in its 5′-upstream region. Gene. 2009, 437: 22-31. 10.1016/j.gene.2009.02.011.
Buzas DM, Robertson M, Finnegan EJ, Helliwell CA: Transcription-dependence of histone H3 lysine 27 trimethylation at the Arabidopsis polycomb target gene FLC. Plant J. 2011, 65: 872-881. 10.1111/j.1365-313X.2010.04471.x.
Schmitges FW, Prusty AB, Faty M, Stützer A, Lingaraju GM, Aiwazian J, Sack R, Hess D, Li L, Zhou S, Bunker RD, Wirth U, Bouweester T, Bauer A, Ly-Hartig N, Zhao K, Chan H, Gu J, Gut H, Fischle W, Müller J, Thomä NH: Histone methylation by PRC2 is inhibited by active chromatin marks. Mol Cell. 2011, 42: 330-341. 10.1016/j.molcel.2011.03.025.
Yun JY, Tamada Y, Kang YE, Amasino RM: Arabidopsis trithorax-related3/SET domain GROUP2 is required for the winter-annual habit of Arabidopsis thaliana. Plant Cell Physiol. 2012, 53: 834-846. 10.1093/pcp/pcs021.
Mathieu O, Probst AV, Paszkowski J: Distinct regulation of histone H3 methylation at lysines 27 and by CpG methylation in Arabidopsis. EMBO J. 2005, 24: 2783-2791. 10.1038/sj.emboj.7600743.
Vaillant I, Paszkowski J: Role of histone and DNA methylation in gene regulation. Curr Opin Plant Biol. 2007, 10: 528-533. 10.1016/j.pbi.2007.06.008.
Weinhofer I, Hehenberger E, Roszak P, Hennig L, Köhler C: H3K27me3 profiling of the endosperm implies exclusion of polycomb group protein targeting by DNA methylation. PLoS Genet. 2010, 6: e1001152-10.1371/journal.pgen.1001152.
约翰逊Deleris, Stroud H, Bernatavichute Y, E,Klein G, Shubert D, Jacobsen SE: Loss of the DNA methyltransferase MET1 Induces H3K9 hypermethylation at PcG target genes and redistribution of H3K27 trimethylation to transposons in Arabidopsis thaliana. PLoS Genet. 2012, 8: e1003062-10.1371/journal.pgen.1003062.
Simon JA, Kingston RE: Polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Mol Cell. 2013, 49: 808-824. 10.1016/j.molcel.2013.02.013.
Bratzel F, López-Torrejón G, Koch M, Del Pozo JC, Calonje M: Keeping cell identity in Arabidopsis requires PRC1 RING-finger homologs that catalyze H2A monoubiquitination. Curr Biol. 2010, 20: 1853-1859. 10.1016/j.cub.2010.09.046.
Li W, Wang Z, Li J, Yang H, Cui S, Wang X, Ma L: Overexpression of AtBMI1C, a polycomb group protein gene, accelerates flowering in Arabidopsis. PLoS One. 2011, 6: e21364-10.1371/journal.pone.0021364.
Bouyer D, Roudier F, Heese M, Andersen ED, Gey D, Nowack MK, Goodrich J, Renou J-P, Grini PE, Colot V, Schnittger A: Polycomb repressive complex 2 controls the embryo-to-seedling phase transition. PLoS Genet. 2011, 7: e1002014-10.1371/journal.pgen.1002014.
Schubert D, Primavesi L, Bishopp A, Roberts G, Doonan J, Jenuwein T, Goodrich J: Silencing by plant polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J. 2006, 25: 4638-4649. 10.1038/sj.emboj.7601311.
Margueron R, Reinberg D: The polycomb complex PRC2 and its mark in life. Nature. 2011, 469: 343-349. 10.1038/nature09784.
Ellis JG, Tokuhisa JG, Llewellyn DJ, Bouchez D, Singh K, Dennis ES, Peacock WJ: Does the ocs-element occur as a functional component of the promoters of plant genes. Plant J. 1993, 4: 433-443. 10.1046/j.1365-313X.1993.04030433.x.
Chen WQ, Chao G, Singh KB: The promoter of a H2O2-inducible, Arabidopsis glutathioneS-transferase gene contains closely linked OBF- and OBP1-binding sites.Plant J1996, 10:955-966.,
Deng W, Buzas DM, Ying H, Robertson M, Taylor J, Peacock WJ, Dennis ES, Helliwell C: Arabidopsis polycomb repressive complex 2 binding sites contain putative GAGA factor binding motifs within coding regions of genes. BMC Genomics. 2013, 14: 593-10.1186/1471-2164-14-593.
Berger N, Dubreucq B, Roudier F, Dubos C, Lepiniec L: Transcriptional regulation of ArabidopsisLEAFY COTYLEDON2involvesRLE, acis-element that regulates trimethylation of histone H3 at lysine 27. Plant Cell. 2011, 23: 4065-4078. 10.1105/tpc.111.087866.
Heck GR, Perry SE, Nichols KW, Fernandez DE: AGL15, a MADS domain protein expressed in developing embryos. Plant Cell. 1995, 7: 1271-1282. 10.1105/tpc.7.8.1271.
Harding EW, Tang W, Nichols KW, Fernandez DE, Perry SE: Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-Like 15. Plant Physiol. 2003, 133: 653-663. 10.1104/pp.103.023499.
Zheng Y, Ren N, Wang H, Stromberg AJ, Perry SE: Global identification of targets of the Arabidopsis MADS domain protein AGAMOUSLike15. Plant Cell. 2009, 21: 2563-2577. 10.1105/tpc.109.068890.
Wang F, Perry SE: Identification of direct targets of FUSCA3, a key regulator of Arabidopsis seed development. Plant Physiol. 2013, 161: 1251-1264. 10.1104/pp.112.212282.
Bentsink L, Hanson J, Hanhart CJ, Blankestijn-deVries H, Coltrane C, Keizer P: Natural variation for seed dormancy inArabidopsisis regulated by additive genetic and molecular pathways. Proc Natl Acad Sci U S A. 2010, 107: 4264-4269. 10.1073/pnas.1000410107.
Nonogaki H: Seed dormancy and germination-emerging mechanisms and new hypotheses. Frontiers Plant Sci. 2014, 5: 233-10.3389/fpls.2014.00233.
Molitor AM, Bu Z, Yu Y, Shen W-H: Arabidopsis AL PHD-PRC1 complexes promote seed germination through H3K4me3-to-H3K27me3 chromatin state switch in repression of seed developmental genes. PLoS Genet. 2014, 10: e1004091-10.1371/journal.pgen.1004091.
Chanvivattana Y, Bishopp A, Schubert D, Stock C, Moon YH, Sung ZR, Goodrich J: Interaction of polycomb-group proteins controlling flowering in Arabidopsis. Development. 2004, 131: 5263-5276. 10.1242/dev.01400.
Aichinger E, Villar CB, Farrona S, Reyes JC, Hennig L, Kohler C: CHD3 proteins and polycomb group proteins antagonistically determine cell identity in Arabidopsis. PLoS Genet. 2009, 5: e1000605-10.1371/journal.pgen.1000605.
Xu L, Shen WH: Polycomb silencing of KNOX genes confines shoot stem cell niches in Arabidopsis. Curr Biol. 2008, 18: 1966-1971. 10.1016/j.cub.2008.11.019.
De Lucia F, Crevillen P, Jones AM, Greb T, Dean C: A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci U S A. 2008, 105: 16831-16836. 10.1073/pnas.0808687105.
Acknowledgements
The authors would like to thank Drs. Mohamed Fokar, Miyoung Kang and Million Tadege, for critically reading and providing helpful comments on the manuscript. We also thank Ms. Katie Pranger for her careful editing. This work was supported by the Oklahoma Agricultural Experiment Station, a grant from the Samuel Roberts Noble Foundation and an endowment from the Walter Sitlington Foundation to RDA.
Author information
Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visithttps://creativecommons.org/licenses/by/4.0/.
The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/)适用于汽车列车的数据可用le, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Veerappan, V., Chen, N., Reichert, A.I.et al.HSI2/VAL1 PHD-like domain promotes H3K27 trimethylation to repress the expression of seed maturation genes and complex transgenes in Arabidopsis seedlings.BMC Plant Biol14,293 (2014). https://doi.org/10.1186/s12870-014-0293-4
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/s12870-014-0293-4
Keywords
- HSI2
- VAL1
- AGL15
- DOG1
- Transgene silencing
- Seed-maturation
- DNA methylation
- Histone methylation
- H3K27me3
- 5-aza-2′-deoxycytidine