- Research article
- Open Access
- Published:
The strigolactone biosynthesis geneDWARF27is co-opted in rhizobium symbiosis
BMC Plant Biologyvolume15, Article number:260(2015)
Abstract
Background
Strigolactones are a class of plant hormones whose biosynthesis is activated in response to phosphate starvation. This involves several enzymes, including the carotenoid cleavage dioxygenases 7 (CCD7) and CCD8 and the carotenoid isomerase DWARF27 (D27).D27expression is known to be responsive to phosphate starvation. InMedicago truncatulaand rice (Oryza sativa) this transcriptional response requires the GRAS-type proteins NSP1 and NSP2; both proteins are essential for rhizobium induced root nodule formation in legumes. In line with this, we questioned whether MtNSP1-MtNSP2 dependentMtD27regulation is co-opted in rhizobium symbiosis.
Results
We provide evidence that MtD27 is involved in strigolactone biosynthesis inM. truncatularoots upon phosphate stress. Spatiotemporal expression studies revealed that this gene is also highly expressed in nodule primordia and subsequently becomes restricted to the meristem and distal infection zone of a mature nodules. A similar expression pattern was found forMtCCD7andMtCCD8.Rhizobium lipo-chitooligosaccharide (LCO) application experiments revealed that of these genesMtD27is most responsive in an MtNSP1 and MtNSP2 dependent manner. Symbiotic expression ofMtD27requires components of the symbiosis signaling pathway; including MtDMI1, MtDMI2, MtDMI3/MtCCaMK and in part MtERN1. This in contrast toMtD27expression upon phosphate starvation, which only requires MtNSP1 and MtNSP2.
Conclusion
Our data show that the phosphate-starvation responsive strigolactone biosynthesis geneMtD27is also rapidly induced by rhizobium LCO signals in an MtNSP1 and MtNSP2-dependent manner. Additionally, we show thatMtD27is co-expressed withMtCCD7andMtCCD8in nodule primordia and in the infection zone of mature nodules.
Background
Legumes evolved the capacity to live in an intimate endosymbiosis with nitrogen-fixing rhizobium bacteria. To host rhizobia intracellularly, nodules are formed on the root of the plant. These nodules provide optimal physiological conditions to the bacteria to fix atmospheric nitrogen gas into ammonia. Recent studies have suggested a role for strigolactones in rhizobium symbiosis [1–6].Here we focus on expression of the strigolactone biosynthesis geneDWARF27during the legume-rhizobium interaction.
Nodule formation is initiated upon perception of lipo-chitooligosaccharide (LCO) signals excreted by compatible rhizobium bacteria [7].These signals mitotically activate root cortical and pericycle cells, resulting in the formation of a nodule primordium [7,8].Rhizobium LCOs (also known as Nod factors) are also required to initiate an infection process to establish intracellular accommodation of the prokaryotic endosymbiont. This infection process starts in curled root hairs where a tube-like structure is formed intracellularly, which guides the rhizobia to the newly formed nodule primordium. There, the rhizobia are released as organelle-like structures (named symbiosomes), which remain surrounded by a plant-derived membrane. These symbiosomes act as nitrogen-fixing units that provide ammonia to the plant cell in exchange of nutrients [7].
Rhizobium LCO signals are perceived by a specific set of LysM-type receptor kinases at the root epidermis. This activates a signaling cascade that is shared with a more ancient endosymbiosis; that between land plants and arbuscular mycorrhizal fungi [7].This signaling cascade consists of a plasma membrane localized LRR-type receptor kinase (named MtDMI2 inMedicago truncatula), a cation ion channel in the nuclear envelope (MtDMI1 inM. truncatula), and a nuclear localized Ca2+/calmodulin dependent protein kinase (MtCCaMK/MtDMI3 inM. truncatula) [9–11].Downstream of this cascade, CCaMK phosphorylates the transcriptional activator CYCLOPS, which orchestrates symbiotic root nodule development in conjunction with other transcription factors [12].Among these are the GRAS-type transcriptional regulators NSP1 and NSP2 [13–15].Experiments in heterologous systems have revealed that NSP2 can form a heterodimer with NSP1, suggesting a regulatory link between both proteins [16].Both transcriptional regulators are essential for rhizobium symbiosis, but also promote mycorrhizal infection [17–20].
Under non-symbiotic conditions, NSP1 and NSP2 regulate the expression ofDWARF27(D27), which encodes a key enzyme in strigolactone biosynthesis [17,21].Strigolactones are a class of plant hormones derived from all-trans-β-carotene [22–24].They are produced mainly in the root of the plant and the biosynthesis of their basic structure involves at least three plastid-localized enzymes; the carotenoid isomerase D27 and the carotenoid cleavage dioxygenases 7 (CCD7) and CCD8 [22].The subsequent activity of these three enzymes results in biosynthesis of carlactone, a precursor of strigolactones. The conversion of carlactone to strigolactones is not completely resolved yet, but there is strong evidence to support that this involves a cytochrome P450 enzyme, encoded byMAX1in Arabidopsis (Arabidopsis thaliana) and rice (Oryza sativa) [25–28].This biosynthetic pathway is under the control of a nutrient sensing mechanism and/or the nutrient status of the plant [29–31].Especially in plants grown under phosphate-limited conditions,D27expression and subsequent strigolactone production is markedly increased in an NSP1/NSP2-dependent manner [17,29].
Under phosphate limitation, strigolactones are exuded into the rhizosphere to attract arbuscular mycorrhizal fungi [31–34].These obligatory biotrophic (symbiotic) fungi can sense strigolactones and respond with an increased hyphal branching, thereby, promoting colonization of plant roots [32,35,36].The endomycorrhizal symbiosis facilitates nutrient exchange between fungus and plant. Mycelium that remains in the soil markedly increases the plant root capacity to access nutrients, especially immobile phosphates. The plant receives these nutrients from the fungi at the expense of carbohydrates [37].Although strigolactones are not essential for establishment of an endomycorrhizal symbiosis, they contribute significantly to increasing root infection levels [33].
Several reports suggest also a role for strigolactones in legume nodule formation. InMedicago sativaandM. truncatula, application of 0.1 μM GR24 was shown to promote nodule formation, whereas slightly higher concentrations inhibited nodule formation inM. truncatula[1,5].In pea (Pisum sativum) andLotus japonicus, strigolactone deficient mutants were shown to produce less nodules, which could be rescued by external application of GR24 [2–4].Furthermore, inM. truncatularoot hairsMtD27andMtCCD8expression is increased at 5 days post rhizobium inoculation [6].This implies thatMtD27expression is regulated also in a symbiotic context. However, the precise regulatory network remains unknown.
Here, we show that inM. truncatula MtD27expression is induced by rhizobium LCOs in an MtNSP1 and MtNSP2-dependent manner, similar as found for the induction ofMtD27by phosphate starvation. However, only induction ofMtD27by rhizobium LCOs requires the symbiosis signaling cascade. Using promoter-reporter constructs, we show thatMtD27is expressed throughout nodule formation. After early activation in the epidermis, its expression becomes restricted to the nodule primordium and subsequently to the nodule meristem and infection zone. Furthermore, we show that in nodule primordia and mature nodulesMtD27is co-expressed withMtCCD7andMtCCD8.
Results
MtD27 is involved in strigolactone biosynthesis
TheM. truncatula D27ortholog (Medtr1g471050) was identified previously [17].By searching theM. truncatulagenome annotation Mt4.0, we noted the presence of 3 close homologs of the originalMtD27gene. To get insight in the relation of these genes toMtD27, we conducted a phylogenetic analysis based on an alignment of D27(-like) proteins of different plant species, including rice and Arabidopsis (Fig.1). This showed that these sequences grouped in three separate phylogenetic clades (Fig.1), consistent with a previous report [38].Interestingly, the D27 clade contains 2 proteins fromM. truncatula: MtD27 [17] as well as a close homolog Medtr7g095920. This shows thatM. truncatulacontains 2 putativeD27genes that could function redundantly. Expression ofMtD27and subsequent strigolactone production is known to be induced by phosphate starvation [17].To get first insight whether Medtr7g095920 may also have a function in strigolactone production inM. truncatularoots, we examined its expression pattern. Analysis of publically available microarray data showed that Medtr7g095920 expression is relatively low, and has limited overlap with expression ofMtD27[39].In roots, Medtr7g095920 expression is not induced by phosphate starvation. This in contrast toMtD27(Additional file1) [17].This suggests that under phosphate limiting conditions onlyMtD27might be involved in strigolactone biosynthesis inM. truncatularoots.
Bayesian phylogeny of D27 and D27-like proteins. Phylogeny was reconstructed based on an alignment of D27 and D27-like proteins from Arabidopsis (At), soybean (Glycine max) (Glyma),Lotus japonicus(Lj),M. truncatula(Medtr), rice (Os), poplar (Populus trichocarpa) (Potri) and grapevine (Vitis vinifera) (VIT). Branch support is indicated by posterior probabilities. Terminals are labeled by their gene name or genbank identifier. Proteins identified fromM. truncatulaare highlighted in bold. The D27 orthology group containing rice OsD27 (gi|2549466546) and Arabidopsis AtD27 (At1G03055) is highlighted in blue. Mid-point rooting was applied for better tree visualization
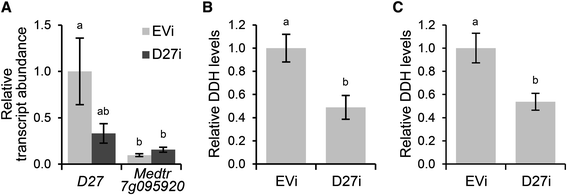
Next, we determined whether MtD27 represents a functional enzyme in the strigolactone biosynthesis pathway. To this end, we generatedM. truncatulacompound plants bearing transgenic roots in whichMtD27expression was reduced through RNAi-mediated knock-down. This reducedMtD27expression by ~65 %, though with substantial variation (Fig.2a). In contrast, expression of Medtr7g095920 was not reduced, but lower thanMtD27(Fig.2a). Measurements of root extracts as well as root exudates collected from theMtD27RNAi roots showed a 45-55 % reduction in strigolactone concentrations compared to that in the empty vector control (Fig.2b and c). Taken together, this shows that MtD27 is involved in strigolactone biosynthesis and represents a functional ortholog of OsD27 from rice.
Effect ofMtD27knock-down on strigolactone biosynthesis.aRelative transcript abundance as determined by qRT-PCR ofMtD27and Medtr7g095920 inM. truncatulatransgenic roots expressing an empty vector control construct (EVi) orMtD27RNAi construct (D27i). Relative transcript abundance was normalized againstMtD27transcript abundance in roots transformed with the empty vector control (EVi).bRelative quantity of the strigolactone didehydro-orobanchol (DDH) (peak area/g FW) in root exudates collected fromM. truncatulatransgenic roots expressing an empty vector control construct (EVi) orMtD27RNAi construct (D27i).cRelative quantity of the strigolactone didehydro-orobanchol (DDH) (peak area/g FW) in root extracts collected fromM. truncatulatransgenic roots expressing an empty vector control construct (EVi) orMtD27RNAi construct (D27i). Data shown represent means of 4-5 biological replicates ± SEM. Different letters above bars indicate statistical difference (p < 0.05, students’t-test)
Expression ofMtD27is increased upon perception of rhizobium LCOs in an MtNSP1 and MtNSP2-dependent manner
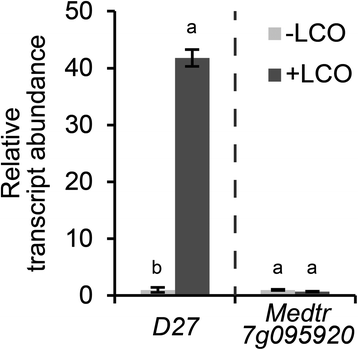
To obtain insight in the symbiotic function ofMtD27, we first determined whether its expression is responsive to rhizobium LCOs. Quantitative RT-PCR (qRT-PCR) reactions on RNA isolated fromM. truncatularoots treated withSinorhizobium melilotiLCOs (~10-9M) for 3 h revealed that expression ofMtD27is strongly induced (Fig.3). To determine whether Medtr7g095920 is also responsive to rhizobium LCOs, we quantified its expression as well. This showed that, unlikeMtD27, Medtr7g095920 is not responsive (Fig.3). The low expression of Medtr7g095920 in combination with its non-responsiveness to phosphate starvation and rhizobium LCOs let us to decide to focus further studies on the symbiotic function ofMtD27.
Relative transcript abundance ofMtD27and Medtr7g095920 after application of rhizobium LCOs. Relative transcript abundance as determined by qRT-PCR ofMtD27and Medtr7g095920 inM. truncatularoot susceptible zones 3 h after mock (-LCO) orS. melilotiLCO (10-9M) (+LCO) treatment. Data shown represent means of 2 biological replicates that each were analyzed in 3-fold (technical replicates) ± SEM. For each gene, transcript abundance was normalized against that of the mock-treated wild type. Different letters above bars indicate statistical difference (p < 0.05, students’t-test)
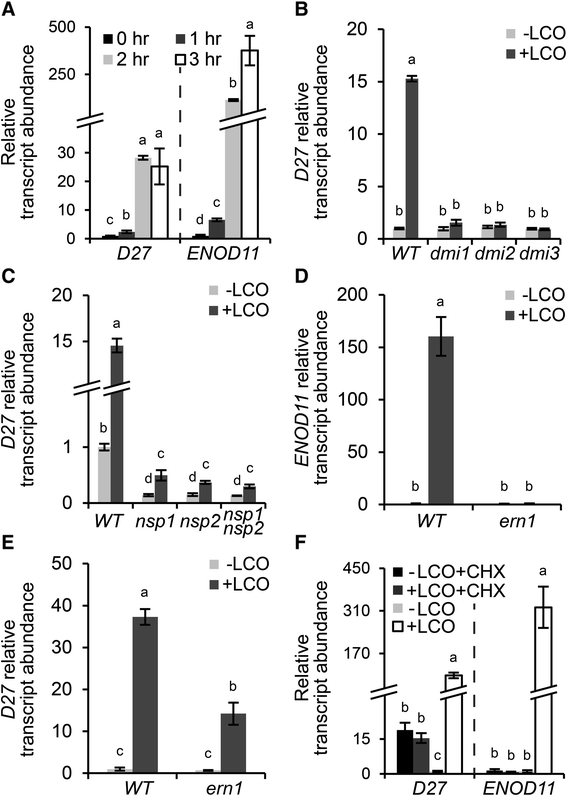
To acquire more insight in the symbiotic responsiveness ofMtD27,我们进行了一个时间序列中存在的实验。To this end,M. truncatulaseedlings were grown in Fåhraeus slides [40], a system optimized to study early responses induced by rhizobium LCOs in the root epidermis.M. truncatularoots of wild-type plants were treated withS. melilotiLCOs (~10-9M) for 0, 1, 2 and 3 h. Subsequently, total RNA was isolated from the so-called susceptible zone, a region of about 1 cm just above the root meristem. Expression analysis by qRT-PCR showed a slight induction ofMtD27already at 1 h post LCO application, and a strong >20-fold induction after 2-3 h compared to mock-treated roots (Fig.4a). This timing and induction level is comparable to that ofMtENOD11; a gene frequently used as marker for rhizobium LCO-induced signaling inM. truncatula[16,41] (Fig.4a).
SymbioticMtD27expression is under direct control ofS. melilotiLCO signaling.aRelative transcript abundance as determined by qRT-PCR ofMtD27andMtENOD11inM. truncatularoot susceptible zones after 0, 1, 2 or 3 h of LCO treatment (10-9M).bRelative transcript abundance as determined by qRT-PCR ofMtD27at 3 h after mock (-LCO) or rhizobium LCO (10-9M) (+LCO) treatment in wild type,Mtdmi1,Mtdmi2andMtdmi3.cRelative transcript abundance as determined by qRT-PCR ofMtD27at 3 h after mock (-LCO) or rhizobium LCO (10-9M) (+LCO) treatment in wild type,Mtnsp1,Mtnsp2andMtnsp1 Mtnsp2.dRelative transcript abundance as determined by qRT-PCR ofMtENOD11at 3 h after mock (-LCO) or rhizobium LCO (10-9M) (+LCO) treatment in wild type andMtern1.eRelative transcript abundance as determined by qRT-PCR ofMtD27at 3 h after mock (-LCO) or rhizobium LCO (10-9M) (+LCO) treatment in wild type andMtern1.fRelative transcript abundance as determined by qRT-PCR ofMtD27andMtENOD11after mock (-LCO) or rhizobium LCO (10-9M) (+LCO) treatment, in presence or absence of 50 μM cycloheximide (CHX). Data shown represent means of 3 biological replicates that each were analyzed in 3-fold (technical replicates) ± SEM. For each gene, transcript abundance was normalized against that of the mock-treated wild type. Different letters above bars indicate statistical difference (p < 0.05, students’t-test)
Induction ofMtENOD11expression by rhizobium LCOs requires a signaling module downstream of LCO perception consisting of MtDMI1, MtDMI2 and MtDMI3/MtCCaMK (the common symbiosis signaling module) and the transcriptional regulators MtNSP1, MtNSP2 and MtERN1 [42–44].To investigate whether induction ofMtD27expression by rhizobium LCOs is also dependent on this signaling module, we conducted qRT-PCR on LCO-susceptible root zones of these mutant plants, usingMtENOD11as control. This revealed thatMtD27expression is not induced inMtdmi1,Mtdmi2andMtdmi3mutant roots (Fig.4b), indicating that symbiotic induction ofMtD27requires the common signaling module genes. Previously, we demonstrated thatMtD27expression in non-inoculated roots is dependent on MtNSP1 and MtNSP2 [17].Here, we found thatMtD27expression is still induced by LCOs in anMtnsp1,Mtnsp2andMtnsp1Mtnsp2mutant background, albeit significantly lower than in wild-type roots (~2-3-fold vs ~15-fold, respectively) (Fig.4c). Next, we monitoredMtD27expression in theMtern1mutant, in which LCO-inducedMtENOD11expression is blocked [45] (Fig.4d). In this mutant, the induction ofMtD27by rhizobium LCOs is about half of that in the wild type (Fig.4e). Taken together, these results show thatMtD27is a rhizobium LCO-responsive gene whose expression in a symbiotic context is largely dependent on the common signaling module and the transcriptional regulators MtNSP1, MtNSP2 and in part MtERN1.
Previously it was shown inVicia sativathat the LCO inducibility of early nodulin genes is indirect, as an inhibition of protein synthesis by cycloheximide (CHX) blocks early nodulin genes expression [46].AsMtD27displays a comparable expression pattern asMtENOD11, we tested whether this gene is a primary target of rhizobium LCO signaling. To this end,M. truncatulaseedlings were grown in Fåhraeus slides for 3 days and pre-treated with 50 μM CHX for 30 min prior to LCO treatment (10–9M, 3 h). The expression ofMtD27andMtENOD11in the susceptible root zone was monitored by qRT-PCR. This showed that in control plantsMtD27expression is elevated by CHX treatment (Fig.4f), suggesting that active protein synthesis is required to keepMtD27expression at basal levels. Additionally it showed that, likeMtENOD11,MtD27expression is induced by rhizobium LCOs in the absence of CHX, but not in the presence of CHX (Fig.4f). This indicates that induction ofMtD27expression by rhizobium LCOs requires new protein synthesis and therefore suggests that induction ofMtD27by rhizobium LCOs is indirect.
MtD27is co-expressed withMtCCD7andMtCCD8during nodule formation
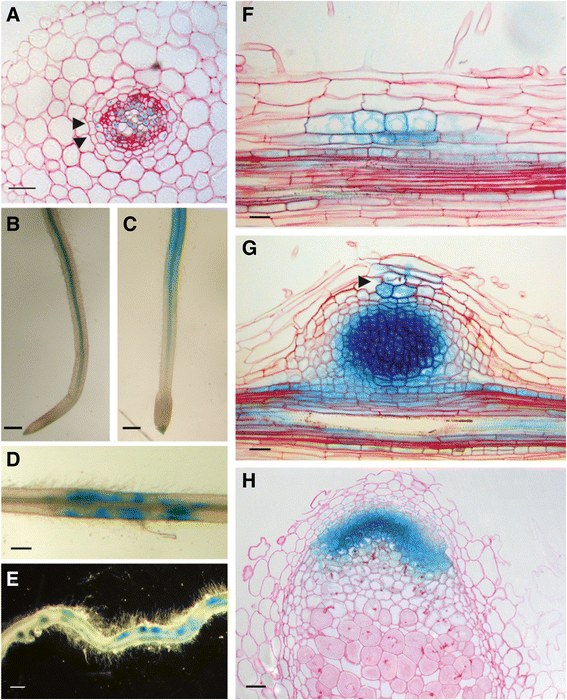
To obtain insight in the symbiotic function ofMtD27, its spatiotemporal expression pattern is analyzed. For this, a ~1 kb fragment representing the 5’ region upstream of the translational start site was cloned into a binary transformation vector in front of a β-glucuronidase (GUS) encoding sequence. This construct was used to createM. truncatulacompound plants carrying transgenic roots. In non-inoculated plants, grown on buffered nodulation medium (BNM) containing no nitrate, but a relatively high phosphate concentration (0.5 mM PO43-),MtD27expression was observed in the vasculature and pericycle (Fig.5a and b). LCO (~10-9M) treatment for 3 h inducedMtD27expression in the root epidermis (Fig.5c).
Spatial expression pattern ofMtD27inM. truncatularoots and nodules. TheMtD27spatial expression pattern was analyzed inM. truncatulatransgenic roots expressing anMtD27promoter-reporter GUS construct.aCross-section through a non-inoculated root. Arrowheads indicate casparian strips, which mark the endodermal cell layer.bMock-treated control root.cRoot treated withS. melilotiLCOs (10-9M) for 3 h.dRoot at four days post inoculation (dpi) withS. melilotistrain 2011.eRoot at 7 dpi.fLongitudinal section through a root at 2 dpi.gLongitudinal section through a nodule primordium (7 dpi). The infection thread is indicated with an arrowhead.hLongitudinal section through an eighteen-day-old nodule. Scale bars are equal to 25 μm (a,fandg), 0.5 mm (b-e) and 50 μm (h). Sections were counterstained with Ruthenium Red
Next, we determined the spatial expression ofMtD27following inoculation withS. melilotistrain 2011. This showed that at 4 days post inoculation (dpi), thepMtD27::GUStransgenic roots showed a patched GUS staining, which was associated with rhizobium root hair infections (Fig.5d). At 7 dpi, the expression in the root ceased, but GUS activity accumulated in nodule primordia (Fig.5e). Sectioning nodule primordia revealed expression ofpMtD27::GUSin dividing pericycle and cortical cells (Fig.5f). This expression maintains in the developing nodule primordium (Fig.5 g), but becomes more restricted in the mature nodule, where it is visible only in the meristem and distal infection zone (Fig.5 h). Taken together, the LCO responsiveness and its spatial expression pattern in nodule primordia, nodule meristem and infection zone strongly support a symbiotic function ofMtD27.
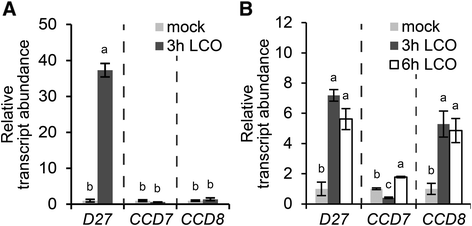
As strigolactone biosynthesis requires at least three enzymes, we tested whether besidesMtD27, alsoCCD7andCCD8are responsive to rhizobium LCOs inM. truncatula. To this end, we first identified the putative orthologs ofCCD7andCCD8from theM. truncatulagenome based on homology to Arabidopsis genes. This revealed that one copy ofCCD7(Medt7g045370;MtCCD7) and two copies ofCCD8(Medtr3g109610 and Medtr7g063800) are encoded in theM. truncatulagenome (Additional file2). Medtr3g109610 was described previously asMtCCD8[6].Analysis of the Medicago gene atlas showed thatMtCCD8and Medtr7g063800 show a similar expression pattern, though expression ofMtCCD8is about 10- to 20-fold higher when compared to Medtr7g063800 [39].This also applies to expression of both genes during phosphate starvation and rhizobium LCO application (Additional file3), and therefore we decided to focus onMtCCD8for the remainder of this study. Expression analysis by qRT-PCR on samples taken from plants grown in Fåhraeus slides showed thatMtCCD7andMtCCD8were not induced at 3 h post rhizobium LCO application (Fig.6a). In contrast, in roots grown on agar-solidified Fähraeus medium supplemented with aminoethoxyvinylglycine (AVG) expression ofMtCCD8is induced ~5-fold 3 h after application of rhizobium LCOs, whereasMtCCD7expression was not substantially affected (Fig.6b).
Expression ofMtD27,MtCCD7andMtCCD8upon treatment with rhizobium LCOs.aRelative transcript abundance as determined by qRT-PCR ofMtD27,MtCCD7andMtCCD8inM. truncatularoot susceptible zones 3 h after mock or rhizobium LCO (10-9米)治疗。RNA was isolated from plants grown in Fåhraeus slides.bRelative transcript abundance as determined by qRT-PCR ofMtD27,MtCCD7andMtCCD8inM. truncatularoot susceptible zones 3 or 6 h after mock or rhizobium LCO (10-9米)治疗。从植物中分离出RNA on agar-solidified Fåhraeus medium supplemented with 1 μM AVG. Data shown represent means of 2-3 biological replicates that each were analyzed in 3-fold (technical replicates) ± SEM. For each gene, transcript abundance was normalized against that of the mock-treated wild type. Different letters above bars indicate statistical difference (p < 0.05, students’t-test)
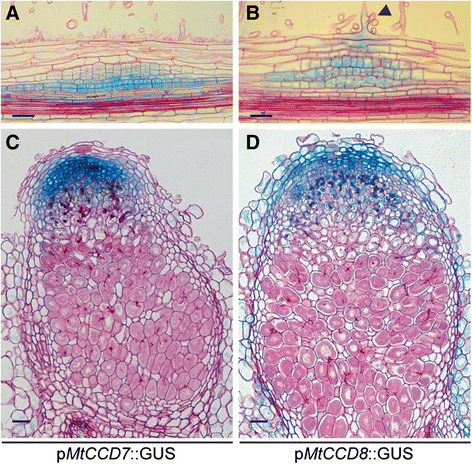
To determine the spatial-temporal expression pattern ofMtCCD7andMtCCD8promoter-reporter GUS constructs were created and introduced inM. truncatularoots usingA. rhizogenes-mediated transformation. We noted a basal expression pattern of both genes in the young root tip, including the susceptible zone and did not observe a discernible change in expression pattern of neitherMtCCD7norMtCCD8following LCO treatment (Additional file4). This suggests that of the MtD27-MtCCD7-MtCCD8 biosynthesis moduleMtD27expression is most strictly controlled in a spatial-temporal manner. However, upon inoculation withS. melilotiit showed that in young (two-day-old) nodule primordiaMtCCD7andMtCCD8are co-expressed withMtD27(Fig.5f,7a and b). Moreover, in mature nodules,MtD27,MtCCD7andMtCCD8are co-expressed in the nodule meristem and distal infection zone (Fig.5 h,7c and d).
Spatial expression pattern ofMtCCD7andMtCCD8inM. truncatulanodule primordia and mature nodules. Expression patterns were analyzed inM. truncatulatransgenic roots expressing promoter-reporter GUS constructs.aLongitudinal section through a root expressing thepMtCCD7::GUSconstruct 2 days post inoculation (dpi) withS. melilotistrain 2011.bLongitudinal section through a root expressing thepMtCCD8::GUSconstruct at 2 dpi. Arrowhead points at an infection thread growing inside the root hair cell.cLongitudinal section through a mature nodule expressing thepMtCCD7::GUSconstruct.dLongitudinal section through a mature nodule expressing thepMtCCD8::GUSconstruct. Scale bars are equal to 50 μm. Sections were counterstained with Ruthenium Red
Previous studies on strigolactone deficient mutants revealed that strigolactones at least in part contribute to root nodule formation and functioning [2–4].To test whetherMtD27function is required for rhizobium symbiosis, we determined the nodulation phenotype ofMtD27RNAi roots. Under these conditions, theMtD27RNAi construct reducedMtD27expression by >90 % (Additional file5a). Examination of theMtD27RNAi roots showed that they can be effectively nodulated (Additional file5b). Sectioning ~40 nodules did not reveal any discernible difference in nodule morphology between nodules formed on control orMtD27RNAi roots (Additional file5c and d). This suggests that either the reduction inMtD27expression is not sufficient to cause a phenotype or thatMtD27is not essential for root nodule development and functioning.
The induction ofMtD27表达式由磷酸剥夺是独立的of the common symbiotic signaling cascade
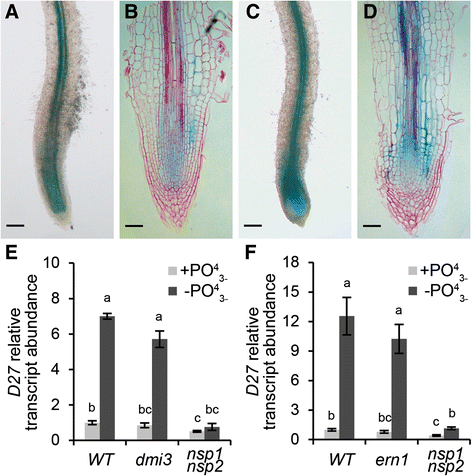
Besides rhizobium LCOs, also phosphate starvation elevatesMtD27expression in an NSP1 and NSP2-dependent manner [17].Induction ofMtD27by rhizobium LCOs requires MtDMI3/MtCCaMK and in part MtERN1. DMI3/CCaMK is positioned directly upstream of NSP1-NSP2 in the LCO signaling pathway [7,47], whereas ERN1 functions in concert with NSP1-NSP2 to regulate expression ofMtENOD11[48].In line with this, we questioned whether MtDMI3/MtCCaMK and MtERN1 are also required to induceMtD27expression in response to a low phosphate status. First, we determined to what extent the spatialMtD27expression inM. truncatularoots is affected by different phosphate regimes. To this end, transgenicM. truncatulaplants carrying theMtD27promoter-GUS reporter construct were grown in perlite for 2 weeks at high phosphate (200 μM PO43-), and subsequently transferred to no phosphate (0 μM PO43-) medium. Plant roots were stained histochemically for GUS activity 5 days after the transfer and compared to control roots. In all plants, GUS staining could be observed in the stele of the roots, as well as in the root apical meristems (Fig.8a and b). Phosphate-starved roots displayed a much more intense staining than control roots, which was most clear in the root apical meristem (Fig.8c and d). However, unlike treatment with rhizobium LCOs, phosphate starvation did not change the spatial expression pattern ofMtD27.
Spatial expression pattern ofMtD27upon phosphate starvation.aTransgenicM. truncatularoot expressingpMtD27::GUSgrown in full nutrient condition (200 μM PO43-).bLongitudinal section of the root shown in (a).cExpression ofpMtD27::GUSinM. truncatulatransgenic roots after 5 days of phosphate starvation (0 μM PO43-).dLongitudinal section of the root shown in (c).eRelative transcript abundance as determined by qRT-PCR ofMtD27in wild type (WT) and theMtdmi3mutant andMtnsp1 Mtnsp2双突变体经过2天的磷酸盐饥饿(0 μM PO43-).fRelative transcript abundance as determined by qRT-PCR ofMtD27in WT and theMtern1mutant andMtnsp1 Mtnsp2双突变体经过2天的磷酸盐饥饿. Scale bars are equal to 250 μm in (a) and (c) and 50 μm in (b) and (d). Sections were counterstained with Ruthenium Red. Data in (e,f) represent means of 3 biological replicates that each were analyzed in 3-fold (technical replicates) ± SEM. For each gene, transcript abundance was normalized against that of the mock-treated wild type. Different letters above bars indicate statistical difference (p < 0.05, students’t-test)
Next, we determined whether MtDMI3/MtCCaMK and MtERN1 are also essential to mediateMtD27expression by phosphate starvation. To this end,M. truncatula Mtdmi3andMtern1knockout mutants were grown in an aeroponic system containing medium with high phosphate (200 μM PO43-) and subsequently transferred to the medium containing no phosphate for 2 days. To determine the expression ofMtD27, qRT-PCR was conducted on RNA isolated from the bottom 2-3 cm of the root. This study reveals that in both mutants induction ofMtD27in response to phosphate deprivation is similar to the induction found in roots of wild-type plants (Fig.8e and f). This indicates that the phosphate response ofMtD27expression is independent of the symbiotic signaling genesMtDMI3/MtCCaMKandMtERN1.
Discussion
几行表明strigolact证据ones play a role in the legume rhizobium symbiosis [1–6].Here, we showed that expression ofMtD27, a gene that is acting in the strigolactone biosynthesis pathway, is strongly elevated by rhizobium LCO-induced signaling. Additionally, we found that this gene is co-expressed withMtCCD7andMtCCD8in nodule primordia as well as in the infection zone of mature nodules. This suggests a putative function for these strigolactone biosynthesis genes during several stages of the legume-rhizobium interaction.
Studies with the strigolactone analog GR24 revealed a nodulation enhancing effect when applied exogenously [1,5].In line with this, a severe reduction in endogenous strigolactone levels due to mutations inccd7orccd8is linked to a moderate decrease in nodulation efficiency [2–4].Foo and Davies [2] conclude that, although strigolactones influence nodule initiation they are not essential. We were unable to confirm these results inMtD27RNAi knockdown roots ofM. truncatula. We cannot rule out that in ourMtD27RNAi experimentsMtD27expression is not sufficiently reduced to cause such moderate nodulation phenotype. Alternatively, phenotypes caused by an altered D27 function may be weaker to that of phenotypes ofccd7orccd8mutants. This hypothesis finds support by studies in arabidopsis and rice, where shoot branching phenotypes were much more severe inccd7andccd8mutants when compared tod27[21,38].Based on this, Waters et al. [38] speculate on residual bioactive compounds present inAtd27mutants. If such a residual bioactive compound also exists inM. truncatula, it is possible that its activity is sufficient for proper nodule initiation and development.
It remains currently unknown how strigolactones promote nodule initiation. One possible mechanism is through promoting the formation of a nodular auxin maximum. Mathematical modelling predicts that such maximum is most likely created through a local reduction in the auxin transport capacity in the root cortex [49].Such reductions in root auxin transport capacity have been observed following rhizobial inoculation [50,51].Strigolactone-deficient mutants of Arabidopsis show elevated auxin transport in both shoots and roots [52].It is proposed that strigolactones act by targeting the PIN auxin-efflux carriers at both the gene expression and protein level [53–57].Therefore, it is possible that rhizobium-induced strigolactone biosynthesis will affect auxin transport and as such contributes to create and/or maintain an auxin maximum during nodule formation. However, it is unlikely that strigolactones alone are sufficient to reduce the auxin transport capacity upon perception of rhizobium LCOs, as strigolactone-deficient mutants still form nodules, although less numerous than wild-type plants [2,3].Possibly, they could function redundantly to another signal, like for example cytokinin [51,58–60] or flavonoids [61].
MtD27expression is elevated within 1-2 h post LCO application, by which it is among the earliest responsive genes. This transcriptional activation is under control of the rhizobium LCO signaling network, which includes MtDMI2, MtDMI1, MtDMI3/MtCCaMK, MtNSP1, MtNSP2 and in part MtERN1. We found that also expression ofMtCCD7andMtCCD8was induced following application of rhizobium LCOs. However, these responses were less pronounced when compared toMtD27.Expression ofMtCCD7was only slightly affected by application of rhizobium LCOs, whereas induction ofMtCCD8was dependent on the growth system. Spatial-temporal expression analysis revealed that in a symbiotic context especiallyMtD27expression is strictly controlled in a spatial-temporal manner. Under non-symbiotic conditionsMtD27is mainly expressed in the stele of the root, whereas rhizobium LCOs activate expression in the root epidermis. Such clear spatial-temporal regulation was not observed forMtCCD7norMtCCD8as both genes have a much broader expression pattern under non-symbiotic conditions. WhyMtD27is strictly controlled under symbiotic conditions remains unknown. Expression ofMtCCD7andMtCCD8might not be rate limiting, or alternatively, induction ofMtD27by rhizobium LCOs might be part of a priming response that prepares epidermal cells for the infection process [6].
Our data hint at a putative role for strigolactones in mature nodules, asMtD27,MtCCD7andMtCCD8are co-expressed in the nodule meristem and distal infection zone. This may suggest that strigolactones promote meristem functioning and/or rhizobial infection. Recently, it was shown thatMtD27andMtCCD8are transcriptionally induced in infected root hairs [6].Our data also indicate expression ofMtD27andMtCCD8in cells that contain growing infection threads in the root nodule, supporting a putative function forMtD27andMtCCD8in the infection process. Furthermore, induction ofMtD27expression by rhizobium LCOs is partly dependent on MtERN1, a transcription factor required for infection thread development [45].
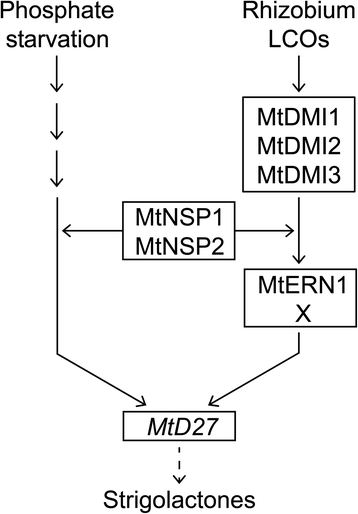
MtD27is also transcriptionally activated by phosphate starvation stress and this induction is dependent on MtNSP1 and MtNSP2 [17].We studied the spatial regulation ofMtD27in response to phosphate starvation and found that the spatial expression pattern remains unchanged, butMtD27expression is increased in the stele and apical root meristem. The transcriptional activation ofMtD27in response to the phosphate status in the environment coincides with an increased exudation of strigolactones [29,30,34].Generally, it is anticipated that this response is contributing to the attraction of endomycorrhizal fungi, which enhance phosphate acquisition from the environment [33,36].We tested whether the induction ofMtD27by phosphate starvation is dependent on the common signaling pathway as well as whether this response is (partially) dependent on MtERN1. Neither signaling components were involved in the phosphate starvation inducedMtD27expression. This indicates that the signaling pathways regulating transcriptional activation ofMtD27by rhizobium LCOs and phosphate starvation only share NSP1 and NSP2 (Fig.9). Interestingly,MtD27transcript abundance is reduced in anMtnsp1Mtnsp2mutant background when compared to wild type [17].Furthermore, we found that induction of this gene upon phosphate stress or LCO signaling still occurs in these mutants, although at very moderate levels (Figs.4eand8f). Taken together, this supports the hypothesis that MtNSP1 and MtNSP2 function in a parallel pathway to facilitate induction ofMtD27by rhizobium LCOs and phosphate starvation stress, rather than being part of the primary signaling cascades [7,15] (Fig.9).
Schematic model depicting the induction ofMtD27expression by phosphate starvation and rhizobium LCO-induced signaling. Rhizobium LCOs activate expression ofMtD27through the common symbiosis signaling module consisting of MtDMI1, MtDMI2 and MtDMI3. Downstream of this module MtERN1 is required for full induction ofMtD27. MtERN1 function is partly redundant, suggesting that MtERN1 might function in conjunction with or redundant to another unknown transcriptional regulator, indicated as X. This unknown factor might be MtERN2, a transcriptional regulator closely related to MtERN1 [48].The pathway leading to activation ofMtD27表达式由磷酸饥饿仍是未知的. The GRAS proteins MtNSP1 and MtNSP2 are required for expression ofMtD27during both phosphate starvation and following rhizobium LCO application. We propose that these proteins function in parallel to both signaling pathways, as previously already suggested for the LCO signaling cascade [7,15]
The D27-CCD7-CCD8 biosynthetic module is largely conserved in higher plants. As we showed thatMtD27is transcriptionally activated in a spatial-temporal manner in response to rhizobium LCOs inM. truncatularoots, this gene may represent an excellent marker gene to study rhizobium-induced signaling in a phylogenetic context.
Conclusions
Here we showed that inM. truncatula,MtD27expression is rapidly increased upon perception of rhizobium LCOs. The gene remains expressed in the dividing cells of the nodule primordium and at subsequent stages its expression becomes confined to the nodule meristem and distal infection zone of the mature nodule. Analysis of the expression ofMtCCD7andMtCCD8showed that they are co-expressed withMtD27in nodule primordia and mature nodules. Additionally, we show that symbiotic expression ofMtD27as well as its expression during phosphate starvation is dependent on the GRAS-type regulators MtNSP1 and MtNSP2. This suggests that the NSP1-NSP2-D27 regulatory unit is co-opted to function in rhizobium symbiosis.
Methods
Plant materials and growth conditions
M. truncatulaJemalong A17,dmi1-1(C71) [9,42],dmi2-1(TR25) [62],dmi3-1(TRV25) [10],nsp1-1(B85) [14,42],nsp2-2(0-4) [13,43],nsp1 nsp2[17] andern1(bit1-1) [45] were used in this study. Plants were grown in a growth chamber at 20 °C under 16/8 photoperiod at 50 μmoles of photons m-2s-1. For gene expression studies, plants were grown in modified Fåhraeus slides [40].一个发芽幼苗被放置在每一个slide, and medium was refreshed every 24 h. Seedlings were grown for 3 days, before subjecting them to a 3 h treatment withSinorhizobium melilotiLCOs (~10-9M), unless stated otherwise. Subsequently, 1 cm root segments were cut just above the root tip and snap-frozen in liquid nitrogen.
For analysis of transcriptional induction ofMtD27by phosphate starvation, plants were grown as previously described [17].This time, plants were grown for ~2.5 weeks before subjecting them to a two-day phosphate starvation regime. Subsequently, 2-3 cm root segments including the root tip were cut and snap-frozen in liquid nitrogen.
Phylogenetic reconstruction
Predicted proteomes ofGlycine max(Wm82.a2.v1) [63],Lotus japonicus(Lj2.5) [64],Medicago truncatula(Mt4.0v1) [65],Oryza sativa(v7.0) [66],Populus trichocarpa(v3.0) [67] andVitis vinifera(Genoscope.12X) [68] were obtained through Phytozome 10 (http://phytozome.jgi.doe.gov/). These proteomes were searched by BLAST usingA. thalianaproteins (TAIR10,www.arabidopsis.org) as query. For phylogenetic reconstruction, full length (predicted) protein sequences were aligned using MAFFT v7.017 [69)中实现Geneious R6 (Biomatters奥克兰, New Zealand), using default parameter settings. After manual inspection, alignments were used for tree building using MrBayes 3.2.2 [70] implemented in Geneious R6, using default parameter settings, with the exception of the rate matrix, for which wag was used. Midpoint rooting was performed for better tree visualization.
Vectors and constructs
For promoter-GUS reporter assays, a ~1 kb (MtD27) or ~2 kb (MtCCD7andMtCCD8) fragment upstream of the translational start site was amplified fromM. truncatulaJemalong A17 genomic DNA using the primers listed in Additional file6. The pMtD27fragment and a β-glucuronidase (GUS)-encoding sequence were recombined into a pDONR-L4L1 and pDONR-L1L2, thereby creating pENTR4-1_pMtD27and pENRT1-2_GUS, respectively. These two constructs were combined with a pENTR2-3_t35S and subsequently recombined into the binary destination vector pKGW-RR-MGW by a multisite gateway reaction (Invitrogen, Carlsbad, USA). The putative promoter fragments ofMtCCD7andMtCCD8were recombined into a pENTR-D-TOPO vector (Invitrogen, Carlsbad, USA), creating pENTR1-2_pMtCCD7and pENTR1-2_pMtCCD8, respectively. Subsequently, both constructs were recombined into pKGWFS7-RR, containing a GUS-GFP fusion reporter, by a single-site gateway reaction (Invitrogen, Carlsbad, USA), creating pKGWFS7-RR_pMtCCD7-GUS and pKGWFS7-RR_pMtCCD8-GUS, respectively.
For RNAi-mediated knockdown ofMtD27, a 268-bp fragment was amplified fromM. truncatulaJemalong A17 root cDNA, using primer pairs MtD27i-F and MtD27i-R (see Additional file6), and cloned into pENTR-D-TOPO (Invitrogen, Carlsbad, USA). TheMtD27被调配到DsRed-modifi RNAi片段ed gateway vector pK7GWIWG2(II)-RR driven by the CaMV35S promoter [71] to obtain the binary construct pK7GWIWG2(II)-RR-p35S-MtD27-RNAi. For the empty vector control, a pENTR containing a ~70 bp multiple cloning site was recombined into pK7GWIWG2(II)-RR to obtain the binary plasmid pK7GWIWG2(II)-RR-p35S-RNAi-control.
All vectors used in this study contain pAtUBQ10::DsRED1as selection marker [72].All cloning vectors are available upon request from Plant Systems Biology (V.I.B.-Ghent University).
Plant transformation and treatments
A. rhizogenes-mediated root transformation ofM. truncatulawas performed as previously described [72].For treatments with rhizobium LCOs, compound plants were transferred to agar-solidified buffered nodulation medium (BNM; 0.9 % Daishin agar (Duchefa, Haarlem, The Netherlands)) [73] containing 1 μM aminoethoxyvinylglycine (AVG) (Sigma, St. Louis, USA). After 3 days, ~100 μl of rhizobium LCOs (~10-9M) was pipetted on top of the root susceptible zone. After 3 h, roots were fixed in 90 % acetone and subsequently stained for GUS activity. For nodulation assays, compound plants were transferred to perlite and watered with Fåhraeus [74] medium without nitrate. One week after transfer, plants were inoculated withS. melilotistrain 2011 (OD600 = 0.05-0.1). For the phosphate starvation experiment, compound plants were transferred into perlite and watered with half-strength Hoagland medium [75].After one week, plants were removed from perlite and washed three times with demineralized water to get rid of the nutrient salts. Plants were re-planted in fresh perlite and watered with half-strength Hoagland medium with (200 μM PO43-) or without (0 μM PO43-) phosphate, respectively. After 5 days, plants were removed from perlite and stained for GUS activity.
Histochemical staining and microtome sectioning
For histochemical GUS staining ofM. truncatularoots and nodules, samples were first rinsed three times with 100 mM phosphate buffer (PBS; pH = 7.2). Samples were transferred to GUS-staining buffer (contains 2 mM K3Fe(CN)6, 2 mM K4Fe(CN)6, 10 mM EDTA, 0.1 % Triton X-100 and 1 mg/ml X-Gluc salt (Duchefa, Haarlem, The Netherlands) in 100 mM PBS, pH = 7.2) and placed under vacuum for 30 min. Next, the samples were incubated in the dark at 37 °C for 3 h. Stained roots were rinsed with PBS (pH = 7.2) three times to stop the reaction.
For historesin embedding, roots and nodule samples were fixed with 5 % glutaraldehyde PBS (pH = 7.2) solution overnight. After fixation, the samples were rinsed with PBS (pH = 7.2) three times and dehydrated through ethanol gradients (20 %, 40 %, 60 %, 80 % and 100 %). Afterwards, the samples were embedded in Technovit 7100 (Heraeus-Kulzer, Wehrheim, Germany), according to the manufacturer’s protocol. GUS-stained samples were sectioned to 7 μm using a microtome (Reichert-Jung, Leica Microsystems, Rijswijk, The Netherlands) and stained with 0.1 % Ruthenium Red for 15 min. Images were taken using a Leica DM5500B microscope equipped with a Leica DFC425C camera (Leica Microsystems, Wetzlar, Germany). Images were digitally processed using Photoshop CS6 (Adobe Systems, San Jose, USA).
qRT-PCR analysis
RNA was isolated from snap-frozen root material using the plant RNA kit (E.Z.N.A. Omega Biotek, Norcross, USA) following the supplier’s manual. cDNA was synthesized from 1 μg total RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, USA). qRT-PCR reactions were set up in a 20 μl reaction system with 2× iQ SYBR Green Super-mix (Bio-Rad, Hercules, USA) and the iQ5 Real-time PCR detecting system according to the manufacturer’s manuals. All primers used in this study were designed using the qPCR settings of Primer3Plus [76].Relative expression values were calculated using the 2-ΔΔCtmethod, usingM. truncatulaubiquitin (MtUBQ10) and polypyrimidine tract-binding protein (MtPTB) as reference genes. Statistical significance was determined based on students’t-test (unpaired, two tailed, equal variance). All primers used in this study are listed in Additional file6.
Strigolactone analysis
M. truncatularoot exudates and root extracts were purified and concentrated as previously described [17,30] with minor modifications. Compound (MtD27RNAi and Empty vector control) plants were grown on perlite and watered twice a week with 50 mL half-strength MS medium (Duchefa, Haarlem, The Netherlands). Seven days prior to strigolactone analysis pots were washed with 3 volumes half-strength MS medium without PO43-(Duchefa, Haarlem, The Netherlands) to initiate phosphate starvation. Strigolactone quantification was performed by comparing retention time and mass transitions with those of an available didehydro-orobanchol standard using ultra-performance LC coupled to MS/MS using [2H6]2’-epi-5-deoxystrigol as an internal standard, as previously described [77].Didehydro-orobanchol MS/MS fragmentation spectra ofM. truncatulawere obtained as previously described [17].Results were subjected to students’t-test (unpaired, two tailed, equal variance).
Medicago gene atlas IDs
Probe IDs used for analysis of gene expression using the Medicago gene expression atlas [39] are listed in Additional file7.
Availability of supporting data
All relevant supporting data can be found within the supplementary files accompanying to this article. The phylogenetic trees were deposited in TreeBase (www.treebase.org) and can be accessed using the following link: (http://purl.org/phylo/treebase/phylows/study/TB2:S18414).
Abbreviations
- BNM:
-
Buffered nodulation medium
- CHX:
-
Cycloheximide
- Dpi:
-
Days post inoculation
- GUS:
-
β-glucuronidase
- LCO:
-
Lipo-chitooligosaccharide
- PBS:
-
Sodium phosphate buffer
- qRT-PCR:
-
Quantitative reverse transcriptase polymerase chain reaction
References
- 1.
Soto MJ, Fernández-Aparicio M, Castellanos-Morales V, García-Garrido JM, Ocampo JA, Delgado MJ, et al. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol Biochem. 2010;42:383–5.
- 2.
Foo E, Davies NW. Strigolactones promote nodulation in pea. Planta. 2011;234:1073–81.
- 3.
Foo E, Yoneyama K, Hugill CJ, Quittenden LJ,里德JB. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol Plant. 2013;6:76–87.
- 4.
Liu J, Novero M, Charnikhova T, Ferrandino A, Schubert A, Ruyter-Spira C, et al. Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J Exp Bot. 2013;64:1967–81.
- 5.
De Cuyper C, Fromentin J, Yocgo RE, De Keyser A, Guillotin B, Kunert K, et al. From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula. J Exp Bot. 2014;66:137–46.
- 6.
Breakspear A, Liu C, Roy S, Stacey N, Rogers C, Trick M, et al. The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell. 2014;26:4680–701.
- 7.
Oldroyd GED. Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol. 2013;11:252–63.
- 8.
Xiao TT, Schilderink S, Moling S, Deinum EE, Kondorosi E, Franssen H, et al. Fate map of Medicago truncatula root nodules. Development. 2014;141:3517–28.
- 9.
Ané J-M, Kiss GB, Riely BK, Penmetsa RV, Oldroyd GED, Ayax C, et al. Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science. 2004;303:1364–7.
- 10.
Lévy J, Bres C, Geurts R, Chalhoub B, Kulikova O, Duc G, et al. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science. 2004;303:1361–4.
- 11.
Charpentier M, Vaz Martins T, Granqvist E, Oldroyd GED, Morris R. The role of DMI1 in establishing Ca2+ oscillations in legume symbioses. Plant Signal Behav. 2013;8:8–10.
- 12.
辛格年代,katz K,兰伯特J, Cerri M, Parniske M。CYCLOPS, a DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe. 2014;15:139–52.
- 13.
Kalo P, Gleason C, Edwards A, Marsh J, Mitra RM, Hirsch S, et al. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science. 2005;308:1786–9.
- 14.
Smit P, Raedts J, Portyanko V, Debelle F, Gough C, Bisseling T, et al. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science. 2005;308:1789–91.
- 15.
Limpens E, Bisseling T. CYCLOPS: a new vision on rhizobium-induced nodule organogenesis. Cell Host Microbe. 2014;15:127–9.
- 16.
Hirsch S, Kim J, Muñoz A, Heckmann AB, Downie JA, Oldroyd GED. GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in Medicago truncatula. Plant Cell. 2009;21:545–57.
- 17.
Liu W, Kohlen W, Lillo A, Op den Camp R, Ivanov S, Hartog M, et al. Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell. 2011;23:3853–65.
- 18.
Maillet F, Poinsot V, André O, Puech-Pagès V, Haouy A, Gueunier M, et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature. 2011;469:58–63.
- 19.
Lauressergues D, Delaux P-M, Formey D, Lelandais-Briere C, Fort S, Cottaz S, et al. The microRNA miR171h modulates arbuscular mycorrhizal colonization of Medicago truncatula by targeting NSP2. Plant J. 2012;72:512–22.
- 20.
Délaux P, Bécard G, Combier J. NSP1 is a component of the Myc signaling pathway. New Phytol. 2013;199:59–65.
- 21.
Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 2009;21:1512–25.
- 22.
Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, et al. The path from beta-carotene to carlactone, a strigolactone-like plant hormone. Science. 2012;335:1348–51.
- 23.
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200.
- 24.
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–94.
- 25.
Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, et al. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell. 2005;8:443–9.
- 26.
Seto Y, Sado A, Asami K, Hanada A, Umehara M, Akiyama K, et al. Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc Natl Acad Sci U S A. 2014;111:1640–5.
- 27.
Cardoso C, Zhang Y, Jamil M, Hepworth J, Charnikhova T, Dimkpa SON, et al. Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proc Natl Acad Sci U S A. 2014;111:2379–84.
- 28.
Zhang Y, van Dijk ADJ, Scaffidi A, Flematti GR, Hofmann M, Charnikhova T, et al. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat Chem Biol. 2014;10:1028–33.
- 29.
Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta. 2007;225:1031–8.
- 30.
López-Ráez JA, Charnikhova T, Gómez-Roldán V, Matusova R, Kohlen W, De Vos R, et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol. 2008;178:863–74.
- 31.
Yoneyama K, Xie X, Kim HI, Kisugi T, Nomura T, Sekimoto H, et al. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta. 2012;235:1197–207.
- 32.
Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–7.
- 33.
Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, et al. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature. 2012;483:341–4.
- 34.
Yoneyama K, Xie X, Kusumoto D, Sekimoto H, Sugimoto Y, Takeuchi Y, et al. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta. 2007;227:125–32.
- 35.
Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, et al. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006;4:1239–47.
- 36.
Yoneyama K, Xie X, Sekimoto H, Takeuchi Y, Ogasawara S, Akiyama K, et al. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 2008;179:484–94.
- 37.
Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol. 2008;6:763–75.
- 38.
Waters MT, Brewer PB, Bussell JD, Smith SM, Beveridge CA. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiol. 2012;159:1073–85.
- 39.
Benedito VA, Torres-Jerez I, Murray JD, Andriankaja A, Allen S, Kakar K, et al. A gene expression atlas of the model legume Medicago truncatula. Plant J. 2008;55:504–13.
- 40.
Heidstra R, Geurts R, Franssen H, Spaink HP, Van Kammen A, Bisseling T. Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol. 1994;105:787–97.
- 41.
Journet EP, El-Gachtouli N, Vernoud V, de Billy F, Pichon M, Dedieu A, et al. Medicago truncatula ENOD11: a novel RPRP-encoding early nodulin gene expressed during mycorrhization in arbuscule-containing cells. Mol Plant Microbe Interact. 2001;14:737–48.
- 42.
Catoira R, Galera C, de Billy F, Penmetsa RV, Journet EP, Maillet F, et al. Four genes of Medicago truncatula controlling components of a nod factor transduction pathway. Plant Cell. 2000;12:1647–66.
- 43.
Oldroyd GED, Long SR. Identification and characterization of nodulation-signaling pathway 2, a gene of Medicago truncatula involved in Nod factor signaling. Plant Physiol. 2003;131:1027–32.
- 44.
Andriankaja A, Boisson-Dernier A, Frances L, Sauviac L, Jauneau A, Barker DG, et al. AP2-ERF transcription factors mediate Nod factor-dependent MtENOD11 activation in root hairs via a novel cis-regulatory motif. Plant Cell. 2007;19:2866–85.
- 45.
Middleton PH, Jakab J, Penmetsa RV, Starker CG, Doll J, Kaló P, et al. An ERF transcription factor in Medicago truncatula that is essential for Nod factor signal transduction. Plant Cell. 2007;19:1221–34.
- 46.
Vijn I, Martinez-Abarca F, Yang W-C, Neves L, Brussel A, Kammen A, et al. Early nodulin gene expression during Nod factor-induced processes in Vicia sativa. Plant J. 1995;8:111–9.
- 47.
Madsen LH, Tirichine L, Jurkiewicz A, Sullivan JT, Heckmann AB, Bek AS, et al. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat Commun. 2010;1:10.
- 48.
Cerri MR, Frances L, Laloum T, Auriac M-C, Niebel A, Oldroyd GED, et al. Medicago truncatula ERN transcription factors: regulatory interplay with NSP1/NSP2 GRAS factors and expression dynamics throughout rhizobial infection. Plant Physiol. 2012;160:2155–72.
- 49.
Deinum EE, Geurts R, Bisseling T, Mulder BM. Modeling a cortical auxin maximum for nodulation: different signatures of potential strategies. Front Plant Sci. 2012;3:96.
- 50.
Mathesius U, Schlaman HR, Spaink HP, Of Sautter C, Rolfe BG, Djordjevic MA. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 1998;14:23–34.
- 51.
Plet J, Wasson A, Ariel F, Le Signor C, Baker D, Mathesius U, et al. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 2011;65:622–33.
- 52.
Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol. 2011;12:211–21.
- 53.
Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol. 2006;16:553–63.
- 54.
Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, et al. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development. 2010;137:2905–13.
- 55.
Shinohara N, Taylor C, Leyser O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 2013;11:e1001474.
- 56.
Sasse J, Simon S, Gübeli C, Liu G-W, Cheng X, Friml J, et al. Asymmetric localizations of the ABC transporter PaPDR1 trace paths of directional strigolactone transport. Curr Biol. 2015;25:647–55.
- 57.
Koltai H. Strigolactones are regulators of root development. New Phytol. 2011;190:545–9.
- 58.
Dello Ioio R, Linhares FS, Sabatini S. Emerging role of cytokinin as a regulator of cellular differentiation. Curr Opin Plant Biol. 2008;11:23–7.
- 59.
Marhavy P, Bielach A, Abas L, Abuzeineh A, Duclercq J, Tanaka H, et al. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev Cell. 2011;21:796–804.
- 60.
Van Zeijl A, Op den Camp RHM, Deinum EE, Charnikhova T, Franssen H, Op den Camp HJM, et al. Rhizobium lipo-chitooligosaccharide signaling triggers accumulation of cytokinins in Medicago truncatula roots. Mol Plant. 2015;8:1213–26.
- 61.
Wasson AP, Pellerone FI, Mathesius U. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell. 2006;18:1617–29.
- 62.
Endre G, Kereszt A, Kevei Z, Mihacea S, Kaló P, Kiss GB. A receptor kinase gene regulating symbiotic nodule development. Nature. 2002;417:962–6.
- 63.
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–83.
- 64.
Sato S, Nakamura Y, Kaneko T, Asamizu E, Kato T, Nakao M, et al. Genome structure of the legume, Lotus japonicus. DNA Res. 2008;15:227–39.
- 65.
Young ND, Debellé F, Oldroyd GED, Geurts R, Cannon SB, Udvardi MK, et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature. 2011;480:520–4.
- 66.
Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, et al. The TIGR Rice Genome Annotation Resource: improvements and new features. Nucleic Acids Res. 2007;35:D883–887.
- 67.
Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science. 2006;313:1596–604.
- 68.
"啊,Aury J-M,诺尔B, Policriti, Clepet C, Casagrande A, et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449:463–7.
- 69.
Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–66.
- 70.
Huelsenbeck JP Ronquist f . MRBAYES:贝叶斯发生rence of phylogenetic trees. Bioinformatics. 2001;17:754–5.
- 71.
Limpens E, Mirabella R, Fedorova E, Franken C, Franssen H, Bisseling T, et al. Formation of organelle-like N2-fixing symbiosomes in legume root nodules is controlled by DMI2. Proc Natl Acad Sci U S A. 2005;102:10375–80.
- 72.
Limpens E, Ramos J, Franken C, Raz V, Compaan B, Franssen H, et al. RNA interference in Agrobacterium rhizogenes-transformed roots of Arabidopsis and Medicago truncatula. J Exp Bot. 2004;55:983–92.
- 73.
Ehrhardt DW, Atkinson EM, Long SR. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science. 1992;256:998–1000.
- 74.
Fåhraeus G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957;16:374–81.
- 75.
Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn. 1950;347(2nd edit):1–32.
- 76.
Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007;35:W71–4.
- 77.
Kohlen W, Charnikhova T, Liu Q, Bours R, Domagalska MA, Beguerie S, et al. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011;155:974–87.
Acknowledgments
We thank Jonathan Kalisvaart and Erik Limpens for their contribution to the project. [2H6]2’-epi-5-deoxystrigol was kindly provided by Harro Bouwmeester. This work was supported by a CAS-KNAW Joint PhD Training Program (06PhD12) (WL), NWO-NSFC Joined Research project (846.11.005) (AvZ, WL, WCY, TB and RG), NWO-VICI (865.13.001) (WK, RG), NWO-VENI (863.15.010) (WK) and the European Research Council (ERC-2011-AdG294790) (TTX, TB).
Author information
Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RG conceived of the study. RG, WCY and TB supervised the study. WK and AvZ performed functional characterization of MtD27. WL and TTX determined the spatiotemporal expression pattern ofMtD27. WL and AvZ determined rhizobium LCO-induced expression ofMtD27. AvZ determined theMtCCD7andMtCCD8spatiotemporal expression patterns. TTX and AvZ determined expression ofMtD27in response to phosphate starvation. AvZ and RG wrote the manuscript. WK, WL, TTX, WCY and TB assisted in editing the manuscript. All authors read and approved the final manuscript.
Arjan van Zeijl, Wei Liu and Ting Ting Xiao contributed equally to this work.
Additional files
Additional file 1:
Relative transcript abundance ofMtD27and Medtr7g095920 upon phosphate starvation.Relative transcript abundance ofMtD27and Medtr7g095920 in roots of plants grown under low (20 μM) or high (2 mM) phosphate conditions. Data were obtained from the Medicago gene atlas [39].Data represent means of three replicates ± SEM. Transcript abundance was normalized againstMtD27转录丰度(2根生长在高mM) phosphate conditions. Different letters above bars indicate statistical difference (p < 0.05, students’t-test). (TIFF 737 kb)
Additional file 2:
Bayesian phylogeny of CCD1, CCD7 and CCD8 proteins.Bayesian phylogeny of CCD1, CCD7 and CCD8 proteins from Arabidopsis (At), soybean (Glycine max) (Glyma),M. truncatula(Medtr), rice (Os), petunia (Petunia hybrida) (Ph), poplar (Populus trichocarpa) (Potri), pea (Pisum sativum) (Ps) and grapevine (Vitis vinifera) (VIT). CCD1 proteins were included as an outgroup. Medicago proteins are highlighted in red. Branch support is indicated by posterior probabilities. Terminals are labeled by their gene or genbank identifier. Mid-point rooting was applied for better tree visualization. (TIFF 3321 kb)
Additional file 3:
Relative transcript abundance ofMtD27,MtCCD7,MtCCD8and Medtr7g063800 during phosphate starvation and upon rhizobium LCO treatment.(a) Transcript abundance ofMtD27,MtCCD7,MtCCD8and Medtr7g063800 in roots of plants grown under low (20 μM) or high (2 mM) phosphate conditions. (b) Transcript abundance ofMtD27,MtCCD7,MtCCD8and Medtr7g063800 in mock-treated roots (-LCO) or roots treated with rhizobium LCOs (+LCO) for 6 or 24 h. Data were obtained from the Medicago gene atlas [39].Data represent means of three replicates ± SEM. Transcript abundance was normalized against 0MtD27转录丰度(2根生长在高mM) phosphate conditions (a) or in the 6 h mock-treated sample (b). Different letters above bars indicate statistical difference (p < 0.05, students’t-test). (TIFF 2450 kb)
Additional file 4:
Spatial expression pattern ofMtCCD7andMtCCD8upon application of rhizobium LCOs.MtCCD7andMtCCD8spatial expression patterns were analyzed inM. truncatulatransgenic roots expressing promoter-reporter GUS constructs. Roots were mock-treated or treated withS. melilotiLCOs (10-9M) for 3 h. Scale bars are equal to 0.5 mm. (TIFF 1705 kb)
Additional file 5:
Nodule phenotype after knock-down ofMtD27through RNAi.(a) Relative transcript abundance as determined by qRT-PCR ofMtD27inM. truncatulatransgenic roots expressing an empty vector control construct (EVi) orMtD27RNAi construct (D27i). (b) Number of nodules formed on plants bearing transgenic roots harboring an empty vector control construct (EVi) orMtD27RNAi construct (D27i). (c) Section through a nodule formed on a root expressing the empty vector control construct. (d) Section through a nodule formed on a root expressing theMtD27RNAi construct. Scale bars are equal to 100 μm. Data shown in (a,b) represent means of 6 (a) or 5 (b) biological replicates ± SEM. Different letters above bars indicate statistical difference (p < 0.05, students’t-test). (TIFF 4222 kb)
Additional file 6:
Primer sequences of all primers used in this study.The recombination sites in the sequences are shown in italic. (CSV 1 kb)
Additional file 7:
Probe IDs used in this study as in the Medicago gene atlas.(CSV 180 bytes)
Rights and permissions
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
van Zeijl, A., Liu, W., Xiao, T.T.et al.The strigolactone biosynthesis geneDWARF27is co-opted in rhizobium symbiosis.BMC Plant Biol15,260 (2015). https://doi.org/10.1186/s12870-015-0651-x
Received:
Accepted:
Published:
Keywords
- DWARF27
- CCD7
- CCD8
- Medicago truncatula
- Nodulation
- Lipo-chitooligosaccharide
- Nod factors
- Rhizobium
- Phosphate starvation
- Strigolactones