- Research article
- Open Access
- Published:
LATE ELONGATED HYPOCOTYL regulates photoperiodic flowering via the circadian clock inArabidopsis
BMC Plant Biologyvolume16, Article number:114(2016)
Abstract
Background
植物不断监控光周期的变化r day length to trigger the flowering cycle at the most appropriate time of the year. It is well established that photoperiodic flowering is intimately associated with the circadian clock inArabidopsis. In support of this notion, many clock-defective mutants exhibit altered photoperiodic sensitivity in inducing flowering. LATE ELONGATED HYPOCOTYL (LHY) and its functional paralogue CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) constitute the core of the circadian clock together with TIMING OF CAB EXPRSSION 1 (TOC1). While it is known that TOC1 contributes to the timing of flowering entirely by modulating the clock function, molecular mechanisms by which LHY and CCA1 regulate flowering time have not been explored.
Results
We investigated how LHY and CCA1 regulate photoperiodic flowering through molecular genetic and biochemical studies. It was found that LHY-defective mutants (lhy-7andlhy-20) exhibit accelerated flowering under both long days (LDs) and short days (SDs). Consistent with the accelerated flowering phenotypes, gene expression analysis revealed that expression of the floral integratorFLOWERING LOCUS T(FT) is up-regulated in thelhymutants. In addition, the expression peaks ofGIGANTEA(GI) andFLAVIN-BINDING, KELCH REPEAT, F-BOX PROTEIN 1(FKF1) genes, which constitute the clock output pathway that is linked with photoperiodic flowering, were advanced by approximately 4 h in the mutants. Furthermore, the up-regulation ofFTdisappeared when the endogenous circadian period is matched to the external light/dark cycles in thelhy-7mutant. Notably, whereas CCA1 binds strongly toFTgene promoter, LHY does not show such DNA-binding activity.
Conclusions
Our data indicate that the advanced expression phases of photoperiodic flowering genes are associated with the clock defects in thelhymutants and responsible for the reduced photoperiodic sensitivity of the mutant flowering, demonstrating that LHY regulates photoperiodic flowering via the circadian clock, similar to what has been shown with TOC1. It is notable that while LHY regulates photoperiodic flowering in a similar manner as with TOC1, the underlying molecular mechanism would be somewhat distinct from that exerted by CCA1 inArabidopsis.
Background
The appropriate timing of flowering is critical for reproductive success in plants. Since the transition from the vegetative to the reproductive phases is irreversible, plants precisely coordinate endogenous developmental signals and environmental cues, such as changes in photoperiod, light quality and quantity, and temperature, to optimize the timing of flowering [1–3]。Both the endogenous and environmental signals are incorporated into flowering genetic pathways via the floral integrators, such asFLOWERING LOCUS T(FT) andSUPPRESSOR OF CONSTANS OVEREXPRESSION 1(SOC1) [4,5]。
Photoperiod is a major environmental cue that profoundly affects floral induction [2,3,6]。Plants monitor photoperiodic changes to anticipate seasonal changes. CONSTANS (CO), which is a B-box zinc finger transcription factor [7], plays a central role in photoperiodic flowering by activatingFTexpression [8,9]。Accordingly, CO-defective mutants and CO-overexpressing plants exhibit photoperiod-insensitive flowering phenotypes [10,11]。
The photoperiod-sensitiveFTinduction is mediated by the distinct accumulation peak of CO in late afternoon under long days (LDs), which is shaped by the coordinated actions of several ubiquitin/proteasome-dependent pathways [12]。A small group of E3 ubiquitin ligases and photoreceptors modulate the CO stability. The light signaling mediator CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1) degrades CO at night [13,14]。In the light phase of the day, two opposing regulations occur through the actions of HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES 1 (HOS1) and FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (FKF1) E3 ubiquitin ligases. HOS1 degrades CO in the morning, and FKF1 stabilizes CO in late afternoon [15,16]。The sequential actions of HOS1 and FKF1 contribute to the maintenance of CO accumulation at a basal level in the morning but at a high level in later afternoon, and thusArabidopsisflowering is induced only under LDs [6]。Meanwhile, PHYTOCHROME B mediates CO degradation, but PHYTOCHROME A and CRYTOCHROME photoreceptors mediate CO stabilization [12]。It is notable that CO accumulation occurs at the specific time phase of the day under LDs, necessitating that photoperiodic flowering would be closely linked with the circadian clock [2,3,17]。
Many clock-defectiveArabidopsismutants exhibit alterations in the photoperiodic sensitivity of flowering time, supporting the intimate linkage between the clock and photoperiodic flowering [18–22]。In addition, the circadian clock regulates the rhythmic expression of photoperiodic flowering genes, such asCO,FKF1,GIGANTEA(GI), andCYCLING DOF FACTOR 1(CDF1) [23–26]。The clock allows the high-level expression ofCOgene occurs in the light only under LDs [23]。The clock-controlled peak ofFKF1andGI表达在下午LD呈现FKF1-GI complex to be formed, which is crucial for CO accumulation [16]。On the other hand, the prevention ofCOandFTexpression by CDFs occurs in the morning through the clock function [26,27]。It has been shown that the early flowering phenotypes of short-period plants, such as TIMING OF CAB EXPRESSION 1 (TOC1)-defective mutant (toc1) and CASEIN KINASE II BETA SUBUNIT 4 (CKB4)-overexpressing plants, are caused by the advanced expression peaks of photoperiodic flowering genes [28,29]。
While the altered flowering phenotypes oftoc1mutant and CKB4-overexpressing plants are entirely caused by clock defects, clock-independent control of photoperiodic flowering by clock components has also been proposed [30–32]。For instance, GI, which plays a role in regulating clock progression [33], activatesFTexpression by directly binding to its gene promoter independent of CO [30]。It has recently been reported that SENSITIVITY TO RED LIGHT REDUCED 1 (SRR1), which is required for normal oscillator function [34], regulates floral transition in a CO-independent manner [31]。Similarly, DE-ETIOLATED 1 (DET1), which is a transcriptional corepressor important for clock progression, acts as a floral repressor [32]。It is notable that GI, SRR1, and DET1 regulate flowering time independent of their roles in the clock function. In addition, they do not require CO, the central promoter of photoperiodic flowering.
LATE ELONGATED HYPOCOTYL (LHY) and its functional paralogue CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) constitute the central oscillator inArabidopsis[20,35,36]。Arabidopsismutants that are defective in LHY and CCA1 exhibit early flowering even under non-inductive conditions [20,22,37]。It has been shown that CCA1 regulates flowering time by binding to theSOC1gene promoter [38]。However, it has not been explored at the molecular level how LHY regulates flowering time.
In this work, with an aim of clarifying the molecular mechanism by which LHY regulates flowering time, we performed molecular genetic and biochemical studies on LHY-defective mutants (lhy-7andlhy-20). Notably, the expression peaks of photoperiodic flowering genes were shifted earlier in thelhymutants. We found that the advanced expression phases of photoperiodic flowering genes are associated with the clock defects in the mutants and underlie the reduced photoperiodic sensitivity of the mutant flowering. By matching the external light/dark cycles to the endogenous circadian period, the early flowering phenotype of the mutants was rescued. Interestingly, CCA1 binds strongly to theFTgene promoter, but LHY does not exhibit such DNA-binding activity. Our data indicate that while LHY regulates the timing of flowering entirely via the circadian clock like TOC1, CCA1 would be functionally distinct from TOC1 and LHY in regulating photoperiodic flowering.
Results
Loss-of-functionlhymutants exhibit early flowering under both LDs and SDs
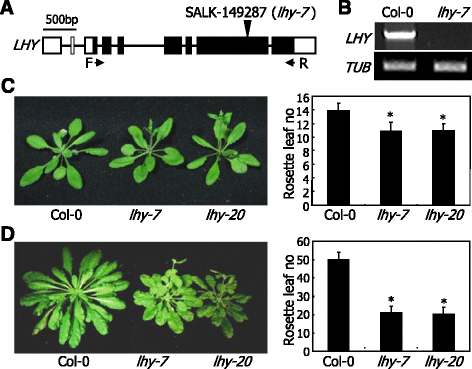
To investigate the functional roles of LHY in photoperiodic flowering, we examined the flowering phenotypes of LHY-defective mutants. We also aimed to clarify the molecular mechanisms by which LHY regulates flowering time: whether LHY affects flowering time entirely by modulating the circadian rhythms like TOC1 [28] or regulating the expression of specific flowering genes like GI [30] or both. In additional to the previously reportedlhy-20mutant [39], we also isolated a T-DNA insertional loss-of-function mutant, which was designatedlhy-7(Fig.1a). Thelhy-7mutant contains a single copy of T-DNA insertion in the sixth exon ofLHYgene. Gene expression analysis confirmed lack ofLHYtranscription in thelhy-7mutant (Fig.1b).
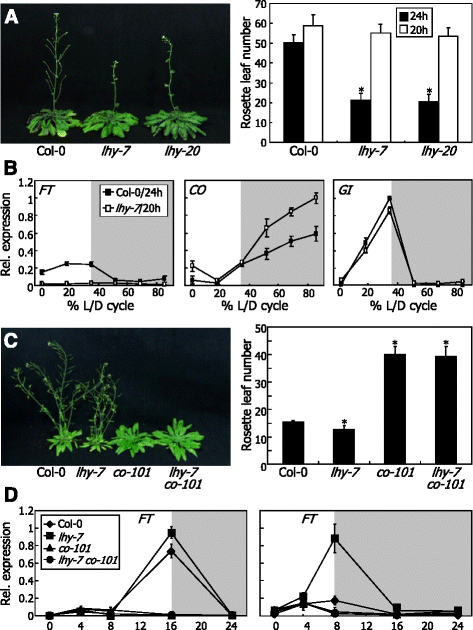
LHY-defective mutants exhibit early flowering under both LDs and SDs.aIsolation of an LHY-defective mutant (lhy-7). It was isolated from a pool of T-DNA insertional lines deposited in theArabidopsisBiological Resource Center (Ohio State University, OH). bp, base pair. F and R, forward and reverse primers, respectively, used to examine the expression ofLHYgene.bLack ofLHYgene expression inlhy-7mutant. Gene expression was examined by RT-PCR. A tubulin gene (TUB) was used as control for constitutive expression.cFlowering phenotypes oflhymutants under LDs. The previously reportedlhy-20mutant was also included in the assays [39]。Plants were grown until flowering in soil under LDs (16-h light and 8-h dark) (left panel). Rosette leaf numbers of 20 plants were averaged and statistically treated using Studentt-test (*P < 0.01) for each plant genotype (right panel).Barsindicate standard error of the mean (SE).dFlowering phenotypes oflhymutants under SDs. Plants were grown until flowering in soil under SDs (8-h light and 16-h dark) (left panel). Flowering times were measured as described in (c) (right panel)
It has been reported that the loss-of-functionlhy-20mutant exhibit a shortening of the circadian period [39,40]。We investigated the circadian period of thelhy-7mutant by examining the rhythmic expression patterns of two representative clock output genes,COLD, CIRCADIAN RHYTHM, AND RNA BINDING 2(CCR2) andCHLOROPHYLL A/B-BINDING PROTEIN 2(CAB2) [41,42]。We found that thelhy-7mutant also exhibits advanced peak expressions of theCCR2andCAB2genes compared to those observed in Col-0 plants (Additional file1), as has been observed with other LHY-defective mutants [20,37,39,40]。
We next examined the flowering phenotypes of thelhy-7andlhy-20mutants under different daylengths by counting the number of rosette leaves at bolting. Both thelhymutants showed accelerated flowering under LDs (Fig.1c). The early flowering phenotypes of the mutants were more prominent under SDs (Fig.1d). The reduced photoperiodic sensitivity of thelhyflowering is similar to what has been observed with LHY-defective mutants in other ecotypes [20,37], showing that the altered flowering phenotypes are associated with LHY function. Since the flowering phenotypes of thelhy-7andlhy-20mutants were similar each other, we chose the former for subsequent molecular assays.
Expression patterns of flowering genes are altered inlhy-7mutant
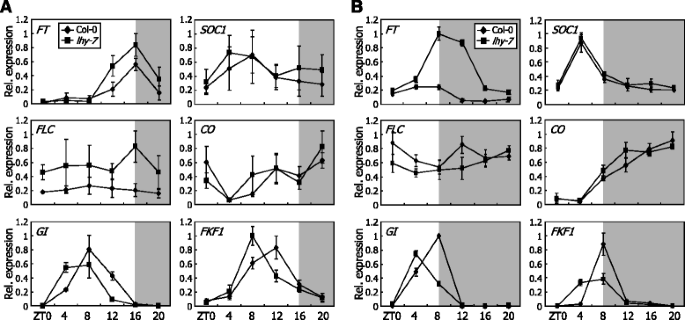
To obtain insights into the molecular mechanism by which LHY regulates flowering time, we analyzed the expression patterns of flowering genes under LDs and SDs. In LD-grown plants, the expression ofFTandSOC1genes was slightly but detectably elevated in thelhy-7mutant (Fig.2a), which is in good agreement with the flowering phenotype of the mutant. Under SDs, the expression ofFTgene, but notSOC1gene, was markedly elevated in thelhy-7mutant (Fig.2b), indicating that theFTinduction is the major cause of the early flowering of the mutant at least under SDs.
Expression patterns of flowering genes are altered inlhy-7mutant.Plants were grown under either LDs or SDs for 10 days on 1/2 X Murashige and Skoog-agar plates (hereafter, referred to as MS-agar plates) before harvesting whole plant materials for total RNA extraction. Transcript levels were examined by quantitative real-time RT-PCR (qRT-PCR). Biological triplicates were averaged and statistically treated using Studentt-test.Barsindicate SE. ZT, zeitgeber time.aExpression of flowering time genes under LDs.bExpression of flowering time genes under SDs
It is known that the circadian clock is closely associated with photoperiodic flowering [1,17,37]。We therefore examined the expression patterns of photoperiodic flowering genes, such asCO,GI, andFKF1, in thelhy-7mutant. The amplitude and waveform ofCOtranscription were not discernibly altered in thelhy-7mutant under both LDs and SDs (Fig.2a and b). In contrast, the waveforms ofGIandFKF1transcription appeared with advanced shifts of the peaks in thelhy-7mutant under both photoperiod regimes. Considering that GI interacts with FKF1 to stabilize CO [16], it seems that the advanced expression phases ofGIandFKF1under SDs would lead to an elevation of the GI-FKF1 complex formation and stabilize CO, underscoring theFTinduction and early flowering in thelhy-7mutant.
We also investigated the expression patterns of flowering genes that belong to other flowering pathways, such as autonomous, thermosensory, and gibberellic acid (GA) pathways, under LDs and SDs. It was found that the expression patterns of autonomous pathway genes, such asFLOWERING LOCUS KH DOMAIN(FLK),FVE, andFCAγ, and GA pathway genes, such asSPYNDLY(SPY) andREPRESSOR OF GA1(RGA1), were not altered in thelhy-7 mutant under both light regimes (Additional file2). In addition, the transcription of two floral repressor genes, which play a role in temperature-responsive flowering, was not significantly affected in the mutant. While the transcript level ofSHORT VEGETATIVE PHASE(SVP) gene in the mutant was comparable to that in Col-0 plants, the transcription ofFLOWERING LOCUS M β(FLMβ) gene was marginally induced in the mutant (Additional file2). Considering the floral repressive activity of FLMβ [43], it is apparent that the early flowering phenotype of thelhy-7mutant is not associated with the slight induction ofFLMβgene.
FLCgene is not related withlhy-7flowering
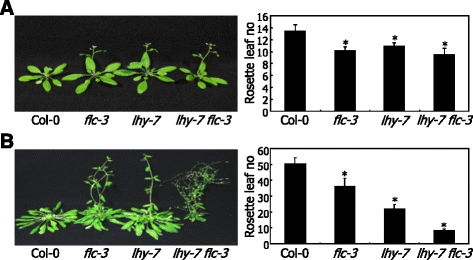
It has been reported that induction of the floral repressorFLOWERING LOCUS C(FLC)是与末花期的表型cca1 lhydouble mutant grown under continuous light conditions [44]。TheFLCgene is also up-regulated in the parentallhymutant in Landsbergerecta(Ler) background. We found thatFLCexpression is slightly increased in thelhy-7mutant under LDs (Fig.2a). In contrast, theFLCexpression was not discernibly elevated in the mutant under SDs (Fig.2b), suggesting thatFLCgene is not associated with the mutant flowering phenotype.
To further examine whetherFLCgene is associated with the flowering phenotype of thelhy-7mutant, we crossed thelhy-7mutant with the FLC-defectiveflc-3mutant that exhibits early flowering, more prominently under SDs [45]。We compared the flowering time of the resultantlhy-7 flc-3double mutant with those of the parental mutants. The flowering phenotype of thelhy-7 flc-3double mutant was not discernibly different from those of the single mutants under LDs (Fig.3a). In contrast, thelhy-7 flc-3double mutant flowered earlier than the single mutants under SDs (Fig.3b), indicating thatFLCgene is not directly associated with the flowering phenotype of thelhy-7mutant.
FLCgene is not associated with the flowering phenotype oflhy-7mutant. Thelhy-7mutant was crossed with theflc-3mutant, resulting inlhy-7 flc-3double mutant. Plants were grown until flowering in soil under either LDs or SDs (left panel). Rosette leaf numbers of 20 plants were averaged and statistically treated using Studentt-test (*P < 0.01) (right panel).Barsindicate SE.aFlowering phenotype oflhy-7 flc-3double mutant under LDs.bFlowering phenotype oflhy-7 flc-3double mutant under SDs
LHY does not bind toFTpromoter
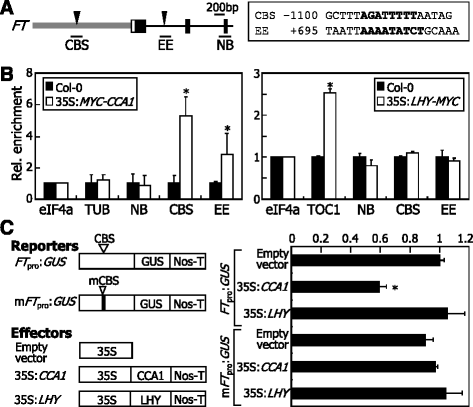
LHY and CCA1 regulate a variety of genes by directly binding to the gene promoters [35,38,46]。For example, the CCA1 transcription factor repressesSOC1expression by binding directly to the gene promoter [38]。We found thatFTgene is significantly up-regulated in thelhy-7mutant (Fig.2). It was therefore suspected that LHY might repressFTexpression perhaps by binding to the gene promoter.
Nucleotide sequence analysis identified a putative CCA1-binding sequence (CBS) in theFTgene promoter and a potential evening element (EE) in the first intron (Fig.4a). To examine whether LHY binds to the CBS and EE sequences, we employed chromatin immunoprecipitation (ChIP) assays using transgenic plants overexpressing aLHY-MYCgene fusion, in which a MYC-coding sequence was fused in-frame to the 3′ end of the LHY-coding sequence (Additional file3). We also included the transgenic plants overexpressing aMYC-CCA1gene fusion in the assays. Both the 35S:LHY-MYCand 35:MYC-CCA1transgenic exhibited elongated hypocotyls, disruption of circadian rhythms, and suppression ofFTexpression (Additional file4), as reported previously [19,47], confirming that the transgenic plants are relevant for ChIP assays. Quantitative ChIP-PCR runs revealed that CCA1 binds to the CBS and EE sequence elements (Fig.4b). In contrast, LHY did not bind to the sequence elements, while it efficiently bound to theTOC1gene promoter containing EE [35]。
LHY does not bind toFTpromoter.aGenomic structure ofFTgene. (Left panel)Gray boxindicates the gene promoter region.Black boxesindicate exons, andwhite boxesindicate untranslated regions. CBS, CCA1-binding sequence. EE, evening element. NB, non-binding sequence. (Right panel) CBS and EE sequences are listed.bChIP assays on binding of CCA1 and LHY toFTpromoter. A MYC-coding sequence was fused in-frame to the 5′ end of the CCA1-coding sequence and the 3′ end of the LHY-coding sequence, and the gene fusions were overexpressed driven by the Cauliflower Mosaic Virus (CaMV) 35S promoter in Col-0 plants, resulting in 35S:MYC-CCA1and 35S:LHY-MYC, respectively. Chromatins were prepared from 7-day-old whole plants grown on MS-agar plates and immunoprecipitated using an anti-MYC antibody. Fragmented genomic DNA was eluted from the protein-DNA complexes and subjected to quantitative PCR. Biological triplicates were averaged and statistically treated using Studentt-test (*P < 0.01).Barsindicate SE. The promoter sequences ofeIF4aandTUBgenes were included as negative controls in the assays. The promoter sequence ofTIMING OF CAB EXPRESSION 1(TOC1) gene containing EE was included as positive control [35]。cSuppression ofFTtranscription by CCA1. The reporter and effector constructs are illustrated (left panel). Transient GUS expression assays were performed usingArabidopsisprotoplasts (right panel). Five measurements were averaged and statistically treated (t-test, *P < 0.01).Barsindicate SE
To verify the binding of CCA1 toFTchromatin, we performed electrophoretic mobility shift assay (EMSA) using a recombinant maltose binding protein (MBP)-CCA1 fusion protein. Consistent with the ChIP data, it was found that CCA1 binds specifically to CBS and EE sequence elements (Additional files5and6).
To examine whether CCA1 binding toFTchromatin influencesFTexpression, we performed transient β-glucuronidase (GUS) expression assays by coexpressing theFTpromoter-driven GUS reporter plasmid (FTpro:GUS) and the effector plasmids (35S:CCA1or 35S:LHY) inArabidopsisprotoplasts. The assays showed that CCA1 negatively regulatesGUSexpression, but LHY does not affectGUSexpression (Fig.4c), consistent with the ChIP data. The repressive effects of CCA1 onGUSexpression disappeared when a reporter plasmid harboring mutations in CBS was coexpressed, indicating that the binding of CCA1 to CBS is essential for the CCA1-mediated repression ofFTexpression. These observations indicate that although LHY and CCA1 are known to be functionally redundant [20,48], LHY is distinct from CCA1 in regulatingFTexpression.
It has been reported thatGIis associated with the flowering phenotype ofcca1 lhydouble mutant [22]。While CCA1 is directly associated withGIpromoter [38,49], it is unknown whether LHY binds toGIpromoter. ChIP assays using transgenic plants overexpressing aLHY-MYCgene fusion showed that LHY also binds toGIpromoter (Additional file7), like CCA1. Binding of both LHY and CCA1 toGIpromoter is in harmony with the repression ofGIexpression in bothCCA1- andLHY-inducible lines [50]。
Clock defects underlie the reduced photoperiodic sensitivity oflhyflowering
LHY is a key component of the central oscillator of plant circadian clock. LHY-defective mutants exhibit a shortened circadian rhythm of approximately 20 h compared to that of Col-0 plants ([39], this work). We found thatlhy-7mutant exhibits early flowering with a reduced sensitivity to photoperiod. Therefore, a critical question was whether the reduced photoperiodic sensitivity of thelhy-7flowering is interconnected with the clock defects in the mutant.
To address the question, we measured the flowering times oflhy-7andlhy-20mutants under light/dark (L/D) cycles that were matched to the endogenous circadian periods of the mutants. If the altered flowering times of thelhymutants are entirely due to the clock defects, the flowering times would be restored by matching the external L/D cycles to the endogenous circadian period, as has been observed with TOC1-defective mutants [21,28]。As expected, we found that the early flowering of thelhymutants was completely annulled under SDs of 20 h (6.7 L: 13.3D) (Fig.5a), which matches to the endogenous period of the mutants ([39], this work), indicating that LHY regulates flowering time entirely via the circadian clock.
Clock defects underlie the reduced photoperiodic sensitivity oflhyflowering.aFlowering times oflhymutants under SDs of differential total duration. Plants were grown until flowering under SDs of 24-h or 20-h total duration. Those grown under SDs of 20-h total duration were photographed (left panel). Rosette leaf numbers of 20 plants were averaged and statistically treated (t-test, *P < 0.01) (right panel).Barsindicate SE.bExpression profiles ofFTand clock output genes inlhy-7mutant grown under SDs of 24-h or 20-h total duration. Transcript levels were examined by qRT-PCR. L/D, light/dark.cFlowering phenotype oflhy-7 co-101double mutant under LDs. Thelhy-7mutant was crossed with theco-101mutant, resulting inlhy-7 co-101double mutant. Plants were grown until flowering in soil under LDs (left panel). Flowering times were measured as described in (a) (right panel).dExpression ofFTgene inlhy-7 co-101double mutant grown under either LDs or SDs. Transcript levels were examined by qRT-PCR. Biological triplicates were averaged.Barsindicate SE
We also analyzed the expression profiles of flowering time genes in thelhy-7mutant under SDs of adjusted L/D cycles. The elevation ofFTtranscript levels was not observed when thelhy-7mutant was grown under SDs of 20 h (Fig.5band Additional file8), as has been observed with short-period plants, such astoc1null mutants [28,29]。It is therefore evident that the reduced photoperiod sensitivity of thelhy-7flowering is caused by the clock defects.
It was found thatCOtranscription was elevated at night under the assay conditions (Fig.5b). Since CO protein is degraded under dark conditions [13,14], it is unlikely that theCOinduction at night is physiologically important for the flowering phenotype of thelhy-7mutant. The waveform ofGItranscription under the adjusted L/D cycles was comparable to that in Col-0 plants under SDs of 24 h. In addition, gene expression assays revealed that the expression of flowering genes, such asFLK,FVE,FCAγ,SVP,SPY,andRGA1, was not altered in thelhy-7mutant under both SDs of 20 and 24 h. The slight induction ofFLMβgene in thelhy-7mutant under SDs of 24 h was also observed under SDs of 20 h (Additional files2and9). On the basis of the flowering times and expression patterns of flowering genes in thelhymutants under adjusted L/D cycles, we concluded that LHY regulates photoperiodic flowering via the clock function.
To verify that LHY regulates flowering time through the clock-controlled CO-FT pathway, we generatedlhy-7 co-101double mutant by crossing thelhy-7mutant with the CO-defectiveco-101mutant. Acceleration of flowering by thelhymutation completely disappeared in thelhy-7 co-101double mutant under LDs (Fig.5c). Accordingly,FTexpression was slightly induced in thelhy-7mutant, but the induction was compromised in thelhy-7 co-101double mutant (Fig.5d). We also examined the level ofFTtranscripts in the single and double mutants under SDs, since the early flowering phenotype of the single mutant was more prominent under this light regime (Fig.1d). We found that the elevation ofFTexpression in thelhy-7mutant was completely annulled in thelhy-7 co-101double mutant (Fig.5d). Together, these observations indicate that LHY-mediated regulation of photoperiodic flowering depends on CO function.
Discussion
LHY-mediated clock control of photoperiodic flowering
植物光周期变化通过集成light signals perceived by the photoreceptors and timing information provided by the circadian clock. InArabidopsis, photoperiod-sensitive induction of the floral integratorFTis a crucial molecular event in photoperiodic flowering [6]。It is known that the coordinated action of light signals and timing information allows the CO transcription factor to accumulate specifically in late afternoon under LDs, which is a prerequisite for the LD-specific induction ofFTgene [1–3,12]。The circadian clock regulates CO activity at both the transcriptional and posttranscriptional levels [12,16,23]。At the transcriptional level, the clock shapes the rhythmic expression patterns ofCOin a way that a high level ofCOtranscripts accumulates during the light phase under LDs [23]。At the posttranscriptional level, the circadian clock contributes to the stabilization of CO in late afternoon under LDs by modulating the expression ofGIandFKF1genes [16,17]。
In this study, we demonstrated that LHY, which is a core component of the central oscillator inArabidopsis, regulates photoperiodic flowering by adjusting the rhythmic expression patterns of photoperiodic flowering genes, such asGIandFKF1. We found that the expression peaks ofGIandFKF1在早些时候基因转移lhy-7mutant, which is consistent with the shortened circadian period of the mutant. A plausible explanation is that the advanced phases ofGIandFKF1expression in thelhy-7mutant would lead to an increase in the formation of GI-FKF1 complexes during the light phase, resulting in a higher-level accumulation of CO in the mutant. In support of this view, the early flowering phenotype of the mutant was completely annulled by matching the external L/D cycles to the endogenous circadian period. Under these assay conditions, the phase shift ofGItranscription was restored and the up-regulated expression ofFTwas suppressed to a basal level in thelhy-7mutant. Together with the previous reports on short-period plants [28,29], it seems that the circadian clock components, including LHY, regulates photoperiodic flowering by adjusting the expression timing of photoperiodic flowering genes.
Common and distinct roles of LHY and CCA1 in photoperiodic flowering
LHY和CCA1 MYB motif-containing transcription factors that function at least in part redundantly in the circadian clock [19,20,48]。Whereas the gain-of-function mutations of bothLHYandCCA1genes disrupt circadian rhythms [19,20], both the LHY-and CCA1-defective mutants exhibit shortened circadian periods [20,37,51]。Thecca1 lhydouble mutants show shorter circadian periods than thecca1orlhysingle mutants [20,37,48]。
On the other hand, there have been some reports supporting distinct roles of CCA1 and LHY. For instance,LHYoverexpression does not enhance pathogen resistance, whereasCCA1overexpression induces pathogen resistance [52,53]。Another example is the differential regulation ofCCA1andLHYexpression by CCA1 HIKING EXPEDITION (CHE) and BROTHER OF LUX ARRHYTHMON (BOA), the components of theArabidopsiscircadian clock. While CHE and BOA bind directly toCCA1gene promoter, they are not associated withLHYgene promoter [46,54]。
We found that CCA1, but not LHY, binds toFTgene promoter to repress its expression. It has been reported that CCA1 repressesSOC1expression by binding to the gene promoter [38]。We observed thatSOC1expression is not discernibly affected bylhymutations, suggesting that LHY is not related withSOC1expression. It is known that LHY and CCA1 form both homodimers and heterodimers in vivo [47,55]。One possibility is that whereas common roles of the two transcription factors would be related with the LHY-CCA1 heterodimers, their distinct roles would be exerted through the homodimers.
Clock-independent control of photoperiodic flowering
It is now apparent that most clock components, including LHY, TOC1, and CKB4, regulate photoperiodic flowering via the clock function [28,29]。Notably, the shorter the circadian periods of clock mutants, the earlier the flowering times of the mutants in most cases [37], supporting that the clock regulates photoperiodic flowering by modulating the rhythmic expression of photoperiodic flowering genes [1,6,17]。
However, there are recent reports supporting that some clock components affect flowering time through clock-independent pathways. CCA1 regulates the expression of floral integrators by directly binding to the gene promoters ([38], this work). GI also binds directly toFTgene promoter [30]。此外,初花期表型的DET1-defective mutants is not restored by matching the external L/D cycles to the endogenous circadian period of the mutants, indicating that DET1 negatively regulates flowering independent of its role in the clock function [32]。Together, it is likely that clock components regulate photoperiodic flowering through both CO-mediated, clock-dependent and CO-free, clock-independent pathways. It will be interesting to investigate how individual clock components regulate flowering time and how the clock-dependent and clock-independent pathways are functionally inter-connected with each other in photoperiodic flowering.
Conclusions
We investigated how LHY regulates photoperiodic flowering by performing molecular genetic and biochemical studies. LHY regulates photoperiodic flowering entirely via the circadian clock. In the LHY-defective mutants, the early flowering phenotypes and the shifted phases of photoperiodic flowering gene expression were recovered by matching the external L/D cycles to the endogenous circadian periods of the mutants. It is notable that the mechanism by which LHY regulates photoperiodic flowering is somewhat distinct from that exerted by CCA1. Our findings would contribute to better understanding of how the clock function is associated with flowering time control in response to photoperiodic signals.
Methods
Plant genotypes and growth conditions
Arabidopsis thalianalines used were in the Columbia (Col-0) background, unless specified otherwise.Arabidopsisplants were grown either in soil or on 1/2 X Murashige and Skoog (MS)-agar plates (hereafter referred to as MS-agar plates) at 23 °C under either LDs (16-h light and 8-h dark) or SDs of 24 h (8 L: 16D) or 20 h (6.7 L: 13.3D) total duration. White light illumination (120 μmol photons m−2s−1) was provided by fluorescent FLR40D/A tubes (Osram, Seoul, Korea).
T-DNA insertional gene knockout mutantslhy-20,flc-3, andco-101have been described previously [39,45,56]。TheLHY-deficientlhy-7mutant (SALK-149287) was isolated from a T-DNA insertional mutant pool deposited in theArabidopsisInformation Resource (TAIR, Ohio State University, OH). Homozygotic lines were obtained by selection for three or more generations and analysis of segregation ratios. Lack of gene expression in the mutants was verified by RT-PCR before use.
A MYC-coding sequence was fused in-frame to the 5′ end of the CCA1-coding sequence or to the 3′ end of the LHY-coding sequence, and the gene fusions were subcloned into the pBA002 vector under the control of the Cauliflower Mosaic Virus (CaMV) 35S promoter. The expression constructs were transformed into Col-0 plants, resulting in 35S:MYC-CCA1and 35S:LHY-MYC, respectively. Overexpression of the transgenes was verified by quantitative real-time RT-PCR (qRT-PCR).
Gene expression assay
Extraction of total RNA samples from appropriate plant materials and qRT-PCR conditions have been described previously [57]。Total RNA samples were pretreated with RNase-free DNase to eliminate contaminating genomic DNA before use.
RNA sample preparation, reverse transcription, and quantitative PCR were conducted according to the criteria that have been proposed to ensure reproducible and accurate measurements [58]。
存在反应是表现在96年好with an Applied Biosystems 7500 Real-Time PCR System (Foster City, CA) using the SYBR Green I master mix in a reaction volume of 20 μl. The PCR primers were designed using the Primer Express Software installed in the system and listed in Additional file10. The two-step thermal cycling profile employed was 15 s at 94 °C and 1 min at 68 °C. AneIF4Agene (At3g13920) was included in the reactions as internal control to normalize the variations in the amounts of cDNA used. All the qRT-PCR reactions were performed in biological triplicates using RNA samples extracted from three independent plant materials grown under identical conditions. The comparative ΔΔCTmethod was employed to evaluate relative quantities of each amplified product in the samples. The threshold cycle (CT) was automatically determined for each reaction using the default parameters of the system. The specificity of PCR reactions was determined by melt curve analysis of the amplified products using the standard method installed in the system.
ChIP assay
ChIP assays were performed, essentially as described previously [59], in biological triplicates using three independent plant materials grown under identical conditions. Seven-day-old 35S:MYC-CCA1and 35S:LHY-MYCtransgenic plants grown on MS-agar plates were vacuum-infiltrated with 1 % (v/v) formaldehyde for cross-linking and ground in liquid nitrogen after quenching the cross-linking process. Chromatin preparations were sonicated into 0.5- to 1-kb fragments. An anti-MYC antibody (Millipore, Billerica, MA) was added to the chromatin solution, which was precleared with salmon sperm DNA/protein G agarose beads (Roche, Indianapolis, IN). The precipitates were eluted from the beads. Cross-links were reversed, and residual proteins were removed by incubation with proteinase K. DNA was recovered using the QIAquick PCR purification kit (Qiagen, Valencia, CA). Quantitative PCR was performed to determine the amounts of genomic DNA enriched in the chromatin preparations. The primers used are listed in Additional file10.
Transient expression assays inArabidopsisprotoplasts
In the reporter vector, a 2-kb promoter sequence ofFTgene was transcriptionally fused to theβ-glucuronidase(GUS)基因。格斯reporter construct harboring a mutated CCA1-binding sequence (CBS) within theFTpromoter was used to investigate the effects of CBS on the binding of CCA1 and LHY to theFTpromoter. The CCA1- and LHY-coding sequences were subcloned under the control of the CaMV 35S promoter in the effector vector. The reporter and effector vectors were cotransfected intoArabidopsismesophyll protoplasts by the polyethylene glycol (PEG)-calcium transfection method [60]。The CaMV 35S promoter-luciferase construct was also cotransfected as internal control. GUS activity was measured by a fluorometric method as described previously [61]。Luciferase activity assay was performed using the Luciferase Assay System kit (Promega, Madison, WI). GUS activities were normalized by luciferase activities.
Flowering time measurement
Plants were grown in soil at 23 °C under either long days of 24 h or short days of 24 h (8 L: 16D) or 20 h (6.6 L: 13.4D) total duration until flowering. Numbers of rosette leaves at bolting were counted, and 20 countings were averaged for each measurement.
Circadian rhythm measurement
Plants were entrained to long day cycles and then transferred to continuous light conditions for 3 days. To trace the circadian rhythm, whole plant materials were harvested at appropriate zeitgeber time (ZT) points for total RNA extraction. Gene transcript levels were measured by qRT-PCR.
Preparation of recombinant MBP-CCA1 fusion protein
A CCA1-coding sequence was subcloned into the pMAL-c2XEscherichia coli(E. coli) expression vector (NEB, Ipswich, MA) harboring a maltose binding protein (MBP)-coding sequence. Recombinant MBP-CCA1 fusion protein was produced inE. coliRosetta2 (DE3) pLysS strain (Novagen, Madison, WI). Harvested cells were resuspended in MBP buffer (20 mM Tris-HCl, pH 7.4, 200 mM NaCl, 1 mM EDTA, 10 mM 2-mercaptoethanol, 1 mM PMSF, and protease inhibitor cocktail (Roche, Indianapolis, IN)). Cell lysates were prepared by running three cycles of freezing and thawing followed by centrifugation. The fusion proteins were affinity-purified as described previously [62]。
EMSA
EMSA was performed using recombinant MBP-CCA1 fusion protein, as described previously [63]。DNA fragments were end-labeled with γ-32P[dATP] using T4 polynucleotide kinase (Takara, Kyoto, Japan). Labeled probes were incubated with 100 ng of MBP or MBP-CCA1 fusion protein for 30 min at room temperature in binding buffer (10 mM Tris-HCl, pH 7.6, 50 mM NaCl, 1 mM EDTA, 5 mM DTT, 5 % glycerol) supplemented with 100 ng poly(dI-dC) in the presence or absence of competitor DNA fragments. The reaction mixtures were resolved on 6 % non-denaturing polyacrylamide gel at 100 V for 1 h. The gels were dried on Whatman 3 MM paper and exposed to X-ray film.
Abbreviations
- BOA:
-
BROTHER OF LUX ARRHYTHMO
- CAB2:
-
CHLOROPHYLL A/B-BINDING PROTEIN 2
- CBS:
-
CCA1-binding sequence
- CCA1:
-
CIRCADIAN CLOCK ASSOCIATED 1
- CCR2:
-
COLD, CIRCADIAN RHYTHM, AND RNA BINDING 2
- CDF1:
-
CYCLING DOF FACTOR 1
- CHE:
-
CCA1 HIKING EXPEDITION
- ChIP:
-
chromatin immunoprecipitation
- CKB4:
-
CASEIN KINASE II BETA SUBUNIT 4
- CO:
-
CONSTANS
- COP1:
-
CONSTITUTIVE PHOTOMORPHOGENIC 1
- DET1:
-
DE-ETIOLATED 1
- EE:
-
evening element
- EMSA:
-
electrophoretic mobility shift assay
- FKF1:
-
FLAVIN-BINDING, KELCH REPEAT, F-BOX PROTEIN 1
- FLC:
-
FLOWERING LOCUS C
- FLK:
-
FLOWERING LOCUS KH DOMAIN
- FLM:
-
FLOWERING LOCUS M
- FT:
-
FLOWERING LOCUS T
- GA:
-
gibberellic acid
- GI:
-
GIGANTEA
- GUS:
-
β-glucuronidase
- HOS1:
-
HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES 1
- LHY:
-
LATE ELONGATED HYPOCOTYL
- L/D:
-
light/dark
- LD:
-
long day
- MBP:
-
maltose binding protein
- RGA1:
-
REPRESSOR OF GA1
- SD:
-
short day
- SOC1:
-
SUPPRESSOR OF CONSTANS OVEREXPRESSION 1
- SPY:
-
SPINDLY
- SVP:
-
SHORT VEGETATIVE PAHSE
- TOC1:
-
TIMING OF CAB EXPRSSION 1
- YFP:
-
yellow fluorescence protein
References
- 1.
Mouradov A, Cremer F, Coupland G. Control of flowering time: interacting pathways as a basis for diversity. Plant Cell. 2002;14:S111–30.
- 2.
Amasino R. Seasonal and developmental timing of flowering. Plant J. 2010;61(6):1001–13.
- 3.
Andrés F, Coupland G. The genetic basis of flowering responses to seasonal cues. Nat Rev Genet. 2012;13(9):627–39.
- 4.
Moon J, Lee H, Kim M, Lee I. Analysis of flowering pathway integrators inArabidopsis. Plant Cell Physiol. 2005;46(2):292–9.
- 5.
Corbesier L, Coupland G. The quest for florigen: a review of recent progress. J Exp Bot. 2006;57(13):3395–403.
- 6.
Song YH, Ito S, Imaizumi T. Flowering time regulation: photoperiod- and temperature-sensing in leaves. Trends Plant Sci. 2013;18(10):575–83.
- 7.
Robson F, Costa MM, Hepworth SR, Vizir I, Piñeiro M, Reeves PH, et al. Functional importance of conserved domains in the flowering-time geneCONSTANSdemonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001;28(6):619–31.
- 8.
An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering ofArabidopsis. Development. 2004;131(15):3615–26.
- 9.
Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, et al. CONSTANS activatesSUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1throughFLOWERING LOCUS Tto promote flowering inArabidopsis. Plant Physiol. 2005;139(2):770–8.
- 10.
Putterill J, Robson F, Lee K, Simon R, Coupland G. TheCONSTANSgene ofArabidopsispromotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80(6):847–57.
- 11.
Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, et al. Distinct roles of CONSTANS target genes in reproductive development ofArabidopsis. Science. 2000;288(5471):1613–6.
- 12.
Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303(5660):1003–6.
- 13.
Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, Deng XW, et al.ArabidopsisCOP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J. 2008;27(8):1277–88.
- 14.
Liu LJ, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, et al. COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering inArabidopsis. Plant Cell. 2008;20(2):292–306.
- 15.
Lazaro A, Valverde F, Piñeiro M, Jarillo JA. TheArabidopsisE3 ubiquitin ligase HOS1 negatively regulates CONSTANS abundance in the photoperiodic control of flowering. Plant Cell. 2012;24(3):982–99.
- 16.
歌YH,史密斯RW, BJ,米勒AJ, Imaizumi t FKF1 conveys timing information for CONSTANS stabilization in photoperiodic flowering. Science. 2012;336(6084):1045–9.
- 17.
Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T. Photoperiodic flowering: time measurement mechanisms in leaves. Annu Rev Plant Biol. 2015;66:441–64.
- 18.
Hicks KA, Millar AJ, Carré IA, Somers DE, Straume M, Meeks-Wagner DR, et al. Conditional circadian dysfunction of theArabidopsis early-flowering 3mutant. Science. 1996;274(5288):790–2.
- 19.
Wang ZY, Tobin EM. Constitutive expression of theCIRCADIAN CLOCK ASSOCIATED 1(CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93(7):1207–17.
- 20.
Mizoguchi T, Wheatley K, Hanzawa Y, Wright L, Mizoguchi M, Song HR, et al. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms inArabidopsis. Dev Cell. 2002;2(5):629–41.
- 21.
Strayer C, Oyama T, Schultz TF, Raman R, Somers DE, Más P, et al. Cloning of theArabidopsisclock geneTOC1, an autoregulatory response regulator homolog. Science. 2000;289(5480):768–71.
- 22.
Mizoguchi T, Wright L, Fujiwara S, Cremer F, Lee K, Onouchi H, et al. Distinct roles ofGIGANTEAin promoting flowering and regulating circadian rhythms inArabidopsis. Plant Cell. 2005;17(8):2255–70.
- 23.
Suarez-Lopez P,惠特利K,罗布森F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering inArabidopsis. Nature. 2001;410(6832):1116–20.
- 24.
Nelson DC, Lasswell J, Rogg LE, Cohen MA, Bartel B.FKF1, a clock-controlled gene that regulates the transition to flowering inArabidopsis. Cell. 2000;101(3):331–40.
- 25.
Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, et al.GIGANTEA: a circadian clock-controlled gene that regulates photoperiodic flowering inArabidopsisand encodes a protein with several possible membrane-spanning domains. EMBO J. 1999;18(17):4679–88.
- 26.
Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS inArabidopsis. Science. 2005;309(5732):293–7.
- 27.
Fornara F, Panigrahi KC, Gissot L, Sauerbrunn N, Rühl M, Jarillo JA, et al.ArabidopsisDOF transcription factors act redundantly to reduceCONSTANSexpression and are essential for a photoperiodic flowering response. Dev Cell. 2009;17(1):75–86.
- 28.
Yanovsky MJ, Kay SA. Molecular basis of seasonal time measurement inArabidopsis. Nature. 2002;419(6904):308–12.
- 29.
Portolés S, Más P. Altered oscillator function affects clock resonance and is responsible for the reduced day-length sensitivity ofCKB4overexpressing plants. Plant J. 2007;51(6):966–77.
- 30.
Sawa M, Kay SA. GIGANTEA directly activatesFlowering Locus TinArabidopsis thaliana. Proc Natl Acad Sci U S A. 2011;108(28):11698–703.
- 31.
Johansson M, Staiger D. SRR1 is essential to repress flowering in non-inductive conditions inArabidopsis thaliana. J Exp Bot. 2014;65(20):5811–22.
- 32.
Kang MY, Yoo SC, Kwon HY, Lee BD, Cho JN, Noh YS, et al. Negative regulatory roles ofDE-ETIOLATED1in flowering time inArabidopsis. Sci Rep. 2015;5:9728.
- 33.
Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh MS, et al. Control of circadian rhythms and photoperiodic flowering by theArabidopsis GIGANTEAgene. Science. 1999;285(5433):1579–82.
- 34.
Staiger D, Allenbach L, Salathia N, Fiechter V, Davis SJ, Millar AJ, et al. TheArabidopsis SRR1gene mediates phyB signaling and is required for normal circadian clock function. Genes Dev. 2003;17(2):256–68.
- 35.
Alabadí D, Oyama T, Yanovsky MJ, Harmon FG, Más P, Kay SA. Reciprocal regulation between TOC1 and LHY/CCA1 within theArabidopsiscircadian clock. Science. 2011;293(5531):880–3.
- 36.
Gendron JM, Pruneda-Paz JL, Doherty CJ, Gross AM, Kang SE, Kay SA.Arabidopsiscircadian clock protein, TOC1, is a DNA-binding transcription factor. Proc Natl Acad Sci U S A. 2012;109(8):3167–72.
- 37.
Niwa Y, Ito S, Nakamichi N, Mizoguchi T, Niinuma K, Yamashino T, et al. Genetic linkages of the circadian clock-associated genes,TOC1,CCA1andLHY, in the photoperiodic control of flowering time inArabidopsis thaliana. Plant Cell Physiol. 2007;48(7):925–37.
- 38.
Lu SX, Webb CJ, Knowles SM, Kim SH, Wang Z, Tobin EM. CCA1 and ELF3 interact in the control of hypocotyl length and flowering time inArabidopsis. Plant Physiol. 2012;158(2):1079–88.
- 39.
Michael TP, Salomé PA, Yu HJ, Spencer TR, Sharp EL, McPeek MA, et al. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science. 2003;302(5647):1049–53.
- 40.
MacGregor DR, Gould P, Foreman J, Griffiths J, Bird S, Page R, et al.HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES1is required for circadian periodicity through the promotion of nucleo-cytoplasmic mRNA export inArabidopsis. Plant Cell. 2013;25(11):4391–404.
- 41.
Carpenter CD, Kreps JA, Simon AE. Genes encoding glycine-richArabidopsis thalianaproteins with RNA-binding motifs are influenced by cold treatment and an endogenous circadian rhythm. Plant Physiol. 1994;104(3):1015–25.
- 42.
Millar AJ, Kay SA. Circadian control ofcabgene transcription and mRNA accumulation inArabidopsis. Plant Cell. 1991;3(5):541–50.
- 43.
Scortecci KC, Michaels SD, Amasino RM. Identification of a MADS-box gene,FLOWERING LOCUS M, that represses flowering. Plant J. 2001;26(2):229–36.
- 44.
Fujiwara S, Oda A, Yoshida R, Niinuma K, Miyata K, Tomozoe Y, et al. Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering inArabidopsis. Plant Cell. 2008;20(11):2960–71.
- 45.
Michaels SD, Amasino RM. Loss ofFLOWERING LOCUS Cactivity eliminates the late-flowering phenotype ofFRIGIDAand autonomous pathway mutations but not responsiveness to vernalization. Plant Cell. 2001;13(4):935–41.
- 46.
Pruneda-Paz JL, Breton G, Para A, Kay SA. A functional genomics approach reveals CHE as a component of theArabidopsiscircadian clock. Science. 2009;323(5920):1481–5.
- 47.
Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carré IA, et al. Thelate elongated hypocotylmutation ofArabidopsisdisrupts circadian rhythms and the photoperiodic control of flowering. Cell. 1998;93(7):1219–29.
- 48.
Lu SX, Knowles SM, Andronis C, Ong MS, Tobin EM. CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock ofArabidopsis. Plant Physiol. 2009;150(2):834–43.
- 49.
Seaton DD, Smith RW, Song YH, MacGregor DR, Stewart K, Steel G, et al. Linked circadian outputs control elongation growth and flowering in response to photoperiod and temperature. Mol Syst Biol. 2015;11(1):776.
- 50.
Knowles SM, Lu SX, Tobin EM. Testing time: can ethanol-induced pulses of proposed oscillator components phase shift rhythms inArabidopsis? J Biol Rhythms. 2008;23(6):463–71.
- 51.
Green RM, Tobin EM. Loss of thecircadian clock-associated protein 1inArabidopsisresults in altered clock-regulated gene expression. Proc Natl Acad Sci U S A. 1999;96(7):4176–9.
- 52.
Wang W, Barnaby JY, Tada Y, Li H, Tör M, Caldelari D, et al. Timing of plant immune responses by a central circadian regulator. Nature. 2011;470(7332):110–4.
- 53.
Burgess A, Searle I. The clock primes defense at dawn. Immunol Cell Biol. 2011;89(6):661–2.
- 54.
Dai S, Wei X, Pei L, Thompson RL, Liu Y, Heard JE, et al. BROTHER OF LUX ARRHYTHMO is a component of theArabidopsiscircadian clock. Plant Cell. 2011;23(3):961–72.
- 55.
Yakir E, Hilman D, Kron I, Hassidim M, Melamed-Book N, Green RM. Posttranslational regulation of CIRCADIAN CLOCK ASSOCIATED1 in the circadian oscillator ofArabidopsis. Plant Physiol. 2009;150(2):844–57.
- 56.
Takada S, Goto K. TERMINAL FLOWER2, anArabidopsishomolog of heterochromatin protein1, counteracts the activation ofFLOWERING LOCUS Tby CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell. 2003;15(12):2856–65.
- 57.
Kwon YJ, Park MJ, Kim SG, Baldwin IT, Park CM. Alternative splicing and nonsense-mediated decay of circadian clock genes under environmental stress conditions inArabidopsis. BMC Plant Biol. 2014;14:136.
- 58.
Udvardi可,Czechowski T, Scheible弯角。十一黄金en rules of quantitative RT-PCR. Plant Cell. 2008;20(7):1736–7.
- 59.
Jung JH, Lee S, Yun J, Lee M, Park CM. The miR172 target TOE3 repressesAGAMOUSexpression duringArabidopsisfloral patterning. Plant Sci. 2014;215–216:29–38.
- 60.
Yoo SD, Cho YH, Sheen J.Arabidopsismesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2(7):1565–72.
- 61.
Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6(13):3901–7.
- 62.
Park J, Kim YS, Kim SG, Jung JH, Woo JC, Park CM. Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination inArabidopsis. Plant Physiol. 2011;156(2):537–49.
- 63.
Seo PJ, Park MJ, Lim MH, Kim SG, Lee M, Baldwin IT, et al. A self-regulatory circuit of CIRCADIAN CLOCK-ASSOCIATED1 underlies the circadian clock regulation of temperature responses inArabidopsis. Plant Cell. 2012;24(6):2427–42.
Acknowledgements
This work was supported by the Leaping Research (NRF-2015R1A2A1A05001636) and Global Research Lab (NRF-2012K1A1A2055546) Programs provided by the National Research Foundation of Korea and the Next-Generation BioGreen 21 Program (PJ0111532015) provided by the Rural Development Administration of Korea. KEG was partially supported by Global PH.D Fellowship Program through the National Research Foundation of Korea (NRF-2015H1A2A1034250).
Availability of supporting data
The data sets supporting the results of this article are included within the article and its additional files.
Authors’ contributions
CMP的构思和设计项目。MJP和YJK performed the molecular and biochemical assays. YJK and KEG measured flowering time of the mutants. CMP, MJP, YJK analyzed the data. CMP and MJP wrote the manuscript. All authors discussed the results and approved the final form of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent to publish
Not applicable.
Ethics
Not applicable.
Author information
Affiliations
Corresponding author
Additional files
Additional file 1:
Circadian rhythms inLHY-defective mutants. Ten-day-old plants grown on ½ X Murashige and Skoog-agar plates (hereafter, referred to as MS-agar plates) under long days (LDs, 16-h light and 8-h dark) were transferred to continuous light conditions at dawn (upper diagram). DAC, days after cold imbibition. Whole plants materials were harvested at the indicated zeitgeber time (ZT) points for total RNA extraction (lower panel). Rhythmic expression ofCHLOROPHYLL A/B-BINDING PROTEIN 2(CAB2) andCAROTENOID AND CHLOROPLAST REGULATION 2(CCR2) genes, which exhibit circadian rhythmic expression patterns [41,42], was examined by quantitative real-time RT-PCR (qRT-PCR). Biological triplicates were averaged. Bars indicate standard error of the mean. TwoLHY-defective mutants (lhy-7andlhy-20) were examined. (PDF 157 kb)
Additional file 2:
Expression of flowering time genes inlhy-7mutant. Plants were grown under either LDs or short days (SDs, 8-h light and 16-h dark) for 10 days on MS-agar plates. Whole plants were harvested at ZT 8 for total RNA extraction. Transcript levels were examined by qRT-PCR. Biological triplicates were averaged and statistically treated using Studentt-test (*P < 0.01). Bars indicate standard error of the mean. (PDF 131 kb)
Additional file 3:
Levels ofLHYandCCA1transcripts in 35S:LHY-MYCand 35S:MYC-CCA1transgenic plants, respectively. Ten-day-old whole plants grown on MS-agar plates under LDs were harvested for total RNA extraction at the indicated ZT points. Transcript levels were examined by qRT-PCR. Biological triplicates were averaged and statistically treated (t-test, *P < 0.01). Bars indicate standard error of the mean. (PDF 121 kb)
Additional file 4:
Functionality of 35S:MYC-CCA1and 35S:LHY-MYCtransgenic plants. A and B. Elongated hypocotyls. Plants were grown on MS-agar plates for 5 days under either LDs (A) or SDs (B). Measurements of 20 seedlings were averaged and statistically treated (t-test, *P < 0.01). Bars indicate standard error of the mean. C. Disruption of circadian rhythms. Expression patterns ofCCR2gene were examined as described in Additional file1. Bars indicate standard error of the mean. D. Suppression ofFTtranscription. Plants were grown under LDs for 10 days on MS-agar plates. Whole plants were harvested at ZT16 for total RNA extraction. Transcript levels were examined as described in Additional file2. Bars indicate standard error of the mean. (PDF 195 kb)
Additional file 5:
Recombinant proteins used for electrophoretic mobility shift assay (EMSA). Recombinant maltose-binding protein (MBP) and MBP-CCA1 fusion protein were prepared inE. colicells and affinity-purified. Protein quality was verified by running on 10 % SDS-PAGE and Coomassie brilliant blue staining. The arrow and arrowhead indicate full-size MBP and MBP-CCA1 proteins, respectively. SM, size marker. kDa, kilodalton. (PDF 263 kb)
Additional file 6:
EMSA on binding of MBP and MBP-CCA1 proteins to conserved sequences inFTlocus. Recombinant MBP and MBP-CCA1 fusion proteins were prepared as described in Additional file5. Radio-labelled CCA1-binding sequence (CBS) and evening element (EE) DNA fragments, which were described in Fig.4a, were used. A. EMSA on MBP binding to DNA fragments. (+) and (−) indicate assays with or without MBP protein. Note that MBP alone does not bind to the CBS and EE sequences. B. EMSA on MBP-CCA1 binding to DNA fragments. The core sequences of CBS and EE were mutated, resulting in mCBS and mEE, respectively. Excess amounts (50X, 100X) of unlabeled DNA fragments were added as competitors. (PDF 182 kb)
Additional file 7:
ChIP assays on LHY binding toGIpromoter. Chromatins were prepared from 7-day-old whole plants grown on MS-agar plates and immunoprecipitated using an anti-MYC antibody. Fragmented genomic DNA was eluted from the protein-DNA complexes and subjected to quantitative PCR. Biological triplicates were averaged and statistically treated using Studentt-test (*P < 0.01). Bars indicate standard error of the mean. GI (NB) amplifies a downstream sequence region ofGIgene, and GI (CBS) amplifies a sequence region containing CBS in theGIpromoter, which has been described previously [38]。(PDF 122 kb)
Additional file 8:
Expression of flowering genes inlhy-7mutant under short days of 20-h total duration. Plants were grown for 10 days under short-day cycles of either 24-h (8-h light and 16-h dark) or 20-h (6.7-h light and 13.3-h dark) total duration. Whole plant materials were harvested throughout the % light-dark (L/D) cycles. Transcript levels were examined by qRT-PCR in Col-0 plants (A) andlhy-7mutant (B). Biological triplicates were averaged. Bars indicate standard error of the mean. h, hour. (PDF 133 kb)
Additional file 9:
Expression of flowering genes inlhy-7mutant under SDs of 20-h total duration. Plants were grown for 10 days under short-day cycles of 20-h total duration (6.7-h light and 13.3-h dark). Whole plant materials were harvested for total RNA extraction. Transcript levels were examined by qRT-PCR. Biological triplicates were averaged and statistically treated using Studentt-test (*P < 0.01). Bars indicate standard error of the mean. (PDF 130 kb)
Additional file 10:
Primers used in qRT-PCR, RT-PCR, and ChIP-qPCR. F, forward primer; R, reverse primer. (PDF 90 kb)
Rights and permissions
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Park, MJ., Kwon, YJ., Gil, KE.et al.LATE ELONGATED HYPOCOTYL regulates photoperiodic flowering via the circadian clock inArabidopsis.BMC Plant Biol16,114 (2016). https://doi.org/10.1186/s12870-016-0810-8
Received:
Accepted:
Published:
Keywords
- Arabidopsis thaliana
- Circadian clock
- Flowering time
- LHY
- Photoperiod