- Research article
- Open Access
- Published:
A cytosolic NAD+-dependent GPDH from maize (ZmGPDH1) is involved in conferring salt and osmotic stress tolerance
BMC Plant Biologyvolume19, Article number:16(2019)
Abstract
Background
Plant glycerol-3-phosphate dehydrogenase (GPDH) catalyzes the reduction of dihydroxyacetone phosphate (DHAP) to produce glycerol-3-phosphate (G-3-P), and plays a key role in glycerolipid metabolism as well as stress responses.
Results
In this study, we report the cloning, enzymatic and physiological characterization of a cytosolic NAD+-dependent GPDH from maize. The prokaryotic expression ofZmGPDH1inE.colishowed that the enzyme encoded byZmGPDH1was capable of catalyzing the reduction of DHAP in the presence of NADH. The functional complementation analysis revealed thatZmGPDH1was able to restore the production of glycerol-3-phosphate and glycerol inAtGPDHc-deficient mutants. Furthermore, overexpression ofZmGPDH1remarkably enhanced the tolerance ofArabidopsisto salinity/osmotic stress by enhancing the glycerol production, the antioxidant enzymes activities (SOD, CAT, APX) and by maintaining the cellular redox homeostasis (NADH/NAD+, ASA/DHA, GSH/GSSG).ZmGPDH1OEArabidopsis植物也降低了叶片失水和展出stomatal aperture under salt and osmotic stresses. Quantitative real-time RT-PCR analyses revealed that overexpression ofZmGPDH1promoted the transcripts accumulation of genes involved in cellular redox homeostasis and ROS-scavenging system.
Conclusions
Together, these data suggested thatZmGPDH1is involved in conferring salinity and osmotic tolerance inArabidopsisthrough modulation of glycerol synthesis, stomatal closure, cellular redox and ROS homeostasis.
Background
It has been shown that glycerol-3-phosphate (G-3-P) serves as a significant intermediary metabolite that connects multiple metabolic pathways, such as gluconeogenes, glycolysis and glycerolipid synthesis [1,2]。Recent evidences proved that G-3-P also plays a crucial role in adapting to adverse stresses, including salinity, pathogenic microbes, freezing and anaerobic stresses [3]。In higher plants, G-3-P can be biosynthesized through two major pathways. In the first route, G-3-P is generated by NAD+-dependent GPDH (EC 1.1.1.8)-mediated reduction of DHAP; while in the second route, G-3-P is produced from glycerol through phosphorylation catalyzed by glycerol kinase (EC 2.7.1.30) [4]。
Multiple forms of GPDH have been identified from eukaryotes, and most of them are proved to be key regulators in stress responses [5,6,7]。There are five GPDH isoforms inArabidopsis, which are associated with different subcellular organelles: one mitochondrial FAD-dependent GPDH (EC 1.1.99.5), two plastidic NAD+-dependent GPDHs and two cytosolic NAD+-dependent GPDHs [5,8,9,10]。Previous studies demonstrated that theAtGPDHc2gene encoded a cytosol-targeted GPDH which is involved in pathogen-elicited defense responses inArabidopsisvia its effects on the provision of G-3-P [5]。Plants deficient in plastid-localized GPDH (SFD1/GLY1)表现出一个严重的障碍在plastidal glycerolipids pathway ofArabidopsisand overexpression ofSFD1/GLY1could increase the plastidic lipid contents as well as the photosynthetic assimilation rate in transgenic rice plants [11]。
In addition to their pivotal role in lipid metabolism, plants GPDHs also participate in modulating the intracellular redox status through the mitochondrial G-3-P shuttle system [8,9]。InArabidopsis thaliana, a mitochondrial FAD-GPDH (EC 1.1.99.5) encoded by the geneAtGPDHm1, along with a cytosolic NAD+-dependent GPDH (EC 1.1.1.8) encoded by the geneAtGPDHc1, was capable of forming the mitochondrial G-3-P shuttle [8]。The operation of G-3-P shuttle is of vital importance to preserve the homeostasis of NADH/NAD+ratio, which is a prerequisite for cells to keep normal metabolic activities. In previous studies, it has been recognized that the expression ofAtGPDHc1andAtGPDHm1is dramatically induced under a variety of stress conditions, like oxygen availability, salinity and dehydration [8,10]。AtGPDHc1knock-out mutants are more sensitive to abscisic acid (ABA) than wild-type (WT) plants, and have failed to stabilize the balance of NADH/NAD+[8]。Loss ofAtGPDHc1also affected other metabolic pathway involved in redox shuttling, such as mitochondrial malate/OAA shuttle [8]。
The characteristics ofGPDHgenes in relation to salinity or osmotic tolerance have been described in some halophilic microalga species [6,12,13]。A putative phosphoserine phosphatase (PSP) domain has been found in GPDH isoforms fromDunaliella salina(DsGPDH2,G3PDH) andChlamydomonas reinhardtii(CrGPD2), which can serve as glycerol-3-phosphatase (EC 3.1.3.2.1) enzyme and directly catalyze the conversion of DHAP to glycerol under high osmotic environment [14,15,16]。Furthermore, the transcription of mushroomGPDHgene is greatly stimulated by drought and salinity conditions; and overexpression ofPsGPDimproves the salinity tolerance of transgenic rice by increasing the osmotic potential and stomatal conductance [17]。
Although the importance ofGPDHgenes in stress responses is well documented in yeast, algae as well as a few plants, there is scarce information about their functions in field crops. Salt and osmotic stresses are the major environmental factors that seriously influence crop growth and productivity [18]。Here, we isolated and characterized a cytosol-localized GPDH (ZmGPDH1) gene from maize, which had functional NAD+-dependent GPDH activity, and apparent transcriptional response to salinity and mannitol treatments. In addition, overexpression ofZmGPDH1inAtGPDHc-deficient mutant and WT lines enhanced tolerance of transgenicArabidopsisto salinity and osmotic stresses, with higher glycerol level, lower fluctuation of cellular redox status and stronger ROS antioxidant defense in comparison to bothatgpdhc2mutant and WT plants. The results showed thatZmGPDH1was pivotal in strengthening salt and osmotic stress tolerance by regulating glycerol production, redox homeostasis and ROS antioxidant defense.
Results
ZmGPDH1encodes a cytosol-targeted protein with NAD+-dependent GPDH activity
One GPDH gene was originally obtained through BLAST searching against the maize genome utilizing the reportedAtGPDHc2 as query [5], the retrieved gene was designated asZmGPDH1. The full length CDS ofZmGPDH1was cloned, which had 458 amino acids and an apparent molecular mass of 51 kDa. The complete CDS sequence ofZmGPDH1was submitted to GeneBank with the following accession number: MH460963. The sequence alignment revealed that ZmGPDH1 exhibited very high protein sequence identity (77%) to AtGPDHc2, and both proteins consisted of one C-terminal GPD domain (PF07479) that represents DHAP-binding site and one N-terminal NAD-binding domain (PF01210), suggesting thatZmGPDH1encodes an NAD+-dependent GPDH (Additional file1: Figure S1).
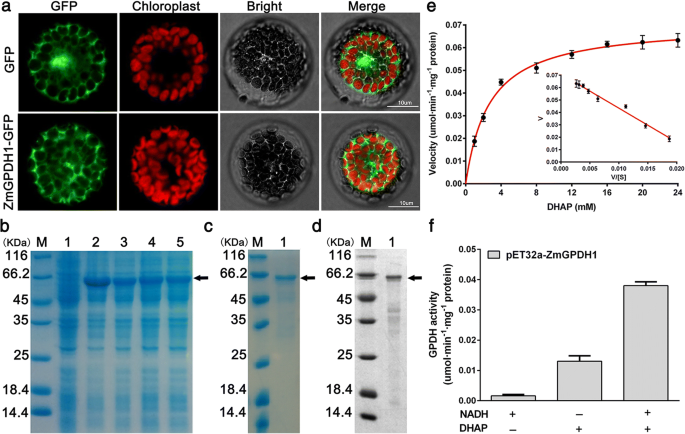
To certify its subcellular localization, the coding region ofZmGPDH1was fused to the N-terminal end ofGFPreporter gene, and the construct was transformed into wild-type (WT)Arabidopsis. The mesophyll protoplasts ofp35S-ZmGPDH1::GFPandp35S::GFP(control) transgenicArabidopsisplants were isolated and monitored (Fig.1a). The free GFP was distributed in cytosol as well as nucleus, whereas the ZmGPDH1-GFP was specifically located in cytosol. Meanwhile, the same result was also found in rice mesophyll protoplasts temporarily expressing the ZmGPDH1-GFP together with the cytosol-marker (mkate protein), which demonstrated that ZmGPDH1 was a cytosol-localized protein (Additional file2: Figure S2).
ZmGPDH1encodes a cytosol-targeted protein with GPDH activities.aSubcellular localization ofZmGPDH1. Confocal microscopy observation ofpBI121-ZmGPDH1::GFPorpBI121-GFP in transgenicArabidopsismesophyll protoplasts. Bars = 10 μm.bCoomassie-stained 12% SDS-PAGE of cell extract from (Lane 1) Rosetta (DE3)Escherichia colistrain and (Lane 2–5) DE3 expressing His-tagged ZmGPDH1 protein.cCoomassie-stained 12% SDS-PAGE of (Lane 1) purified ZmGPDH1 fusion protein.dWestern blot analysis of (Lane 1) purified ZmGPDH1 fusion protein using anti-6 × His antibody as probe.eThe kinetic properties of ZmGPDH1 with regard to DHAP.fGPDH enzyme activities from purified ZmGPDH1 fusion protein. The reaction was performed in the presence (+) and absence (−) of NADH and DHAP, respectively
To further study the catalytic characteristics of ZmGPDH1, the recombinant protein generated by theE. coliRosetta (DE3) strain expressing plasmid of 6 × His taggedZmGPDH1was purified with a Ni-NTA column. A ZmGPDH1-His fusion protein with an expected size of 65 kDa (consisting of target gene and histidine marker) was identified by SDS/PAGE and Western blot (Fig.1b, c and d). The recombinant ZmGPDH1 protein was assayed for its kinetic properties relative to the substrate DHAP. Using Eadie-Hofstee plot, theKmandVmaxof DHAP were estimated as 2.75 mM and 0.071 umol·min− 1·mg− 1protein, respectively (Fig.1e); besides, addition of NADH strongly stimulated the enzyme activity (Fig.1f). These results indicated that the purified ZmGPDH1 protein was able to catalyze the reduction of DHAP with the assistant of NADH. Furthermore, the optimum pH of the enzyme activity was determined to be pH 7.0 and the optimum temperature was 35 °C, respectively (Additional file3: Figure S3).
Expression profiles ofZmGPDH1in response to NaCl or mannitol treatment
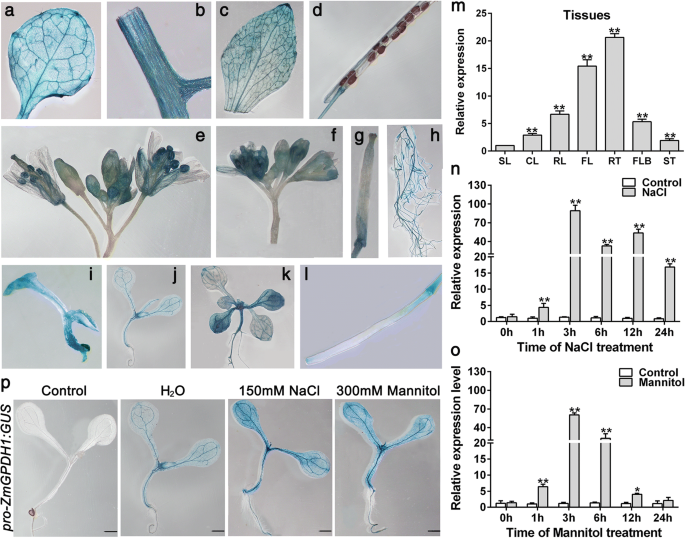
To examine the potential functions ofZmGPDH1in response to plant growth, the promoter region ofZmGPDH1was amplified and fused to N-terminus ofGUSreporter gene, and theproZmGPDH1::GUSconstruct was transformed to WTArabidopsisplants. In 4-day-old transgenic seedlings, high GUS activity was observed in shoots apical meristem; in 7-day-old or 14-day-old seedlings, high GUS activity was observed in young leaves and petioles (Fig.2). In 5-week-oldproZmGPDH1::GUS transgenic plants, strong constitutiveGUSactivity was shown in flowers, roots, rosette leaves, and stems. To further verify these findings, quantitative real-time RT-PCR (qRT-PCR) was carried out, and the level ofGUStranscripts in siliques of 5-week-oldproZmGPDH1::GUStransgenic plants was used as calibrator (Fig.2m). Consistent with the result ofGUSactivity assay, theGUStranscripts could be observed in all tissues examined, with high levels of transcription in roots, flowers and rosette leaves. These results suggested that theZmGPDH1promoter exhibited a tissue-specific expression pattern.
Expression characteristics ofZmGPDH1gene.ZmGPDH1promoter-GUS assay in various tissues, including (a) rosette leaf, (b) stem, (c) cauline leaf, (d) mature silique, (e) flower, (f) flower bud, (g) stigma, (h) root, (i) 4-day-old plant, (j) 7-day-old plant, (k) 14-day-old plant and (l) immature silique. (m) The transcript level ofGUSgene in different tissues of 5-week-oldproZmGPDH1::GUS transgenic plants (T3 generation), including stems (ST), rosette leaves (RL), cauline leaves (CL), roots (RT), flowers (FL), flower buds (FLB), siliques (SL). The expression ofGUSgene in SL was used as a calibrator. The transcriptional response ofZmGPDH1in maize roots exposed to (n) NaCl or (o) mannitol treatments. The expression ofZmGPDH1in untreated samples (control) harvested at each time point was used as a calibrator. (p) The analysis of GUS activity in response to mannitol or NaCl treatment. 7-day-oldproZmGPDH1::GUS transgenic seedlings were transferred to either half-strength MS plates (1/2 MS), plates with 300 mM mannitol or plates with 150 mM NaCl for 12 h before GUS staining. Non-transformed wild-type (WT) was used as a control. Bars = 100 μm. The asterisks represented a significant difference as determined by the Student′s t-test (*P< 0.05, **P< 0.01)
It has been proven thatGPDHgenes are essential for stress adaptations inyeast, marine algae andArabidopsis[5,17,18,19]。Therefore, to explore its possible involvement in stress responses in maize, we first analyzed the transcripts accumulation ofZmGPDH1under different stress treatments. The qRT-PCR results showed thatZmGPDH1was remarkably up-regulated by both salinity and osmotic stresses in maize roots, which reached the highest level at 3 h under both treatments (Fig.2n and o). Notably, the expression ofproZmGPDH1::GUSwas also enhanced in the presence of NaCl and mannitol treatments (Fig.2p), suggested thatZmGPDH1was involved in the transcriptional response during salt or osmotic adaptions.
Overexpression ofZmGPDH1enhanced the tolerance ofArabidopsisto salt and osmotic stresses
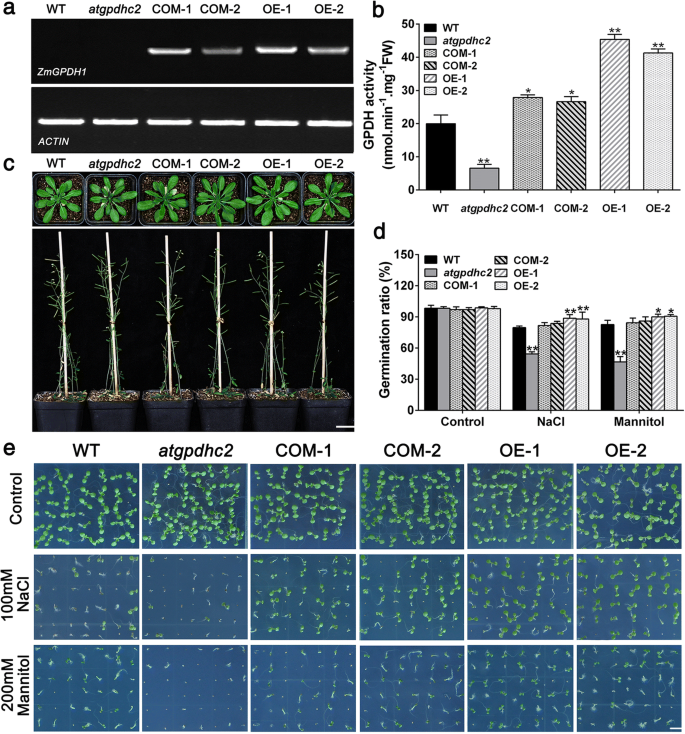
Next, to further understand howZmGPDH1responds to salt or osmotic stress,ZmGPDH1transformedAtGPDHc2-deficient mutant (COM-1, COM-2) and WT (OE-1, OE-2) lines were generated. The T-DNA insertion mutant ofAtGPDHc2PCR和逆转录电脑确认了吗R (RT-PCR), and the homozygousatgpdhc2mutants were selected for the transformation studies (Additional file4: Figure S4). The RT-PCR results showed that the full-length transcription product ofZmGPDH1was absent in the WTArabidopsisoratgpdhc2mutant, but existed in allZmGPDH1transgenic lines (Fig.3a). The enzymatic assay revealed that GPDH activities ofZmGPDH1overexpression lines (OE-1, OE-2) were 2.1- to 2.3-fold higher than that in the WT, while GPDH activities ofatgpdhc2变异的31%,在WT。与此同时,GPDH activities ofZmGPDH1互补线(COM-1 COM-2) 1.2 -1.3-fold higher than that in the WT, which indicated thatZmGPDH1was successfully expressed and functioned with GPDH activity in both OE and COM lines (Fig.3b). Compared with WT,atgpdhc2,COM and OE lines showed no aberrant phenotype under normal growth conditions, no matter in the vegetative growth phase or reproductive developmental stages (Fig.3c).
Germination characteristics of theZmGPDH1transgenic lines in response to salt or osmotic stresses.aReverse transcription PCR (RT-PCR) ofZmGPDH1transcripts in the WT,atgpdhc2,ZmGPDH1transformedatgpdhc2(COM-1, COM-2) and transgenic WT (OE-1, OE-2) plants.ACTINserved as the internal reference.bThe GPDH activities inZmGPDH1transgenic lines compared with the WT andatgpdhc2.cMorphological comparison among the WT,atgpdhc2, COM-1, COM-2, OE-1 and OE-2 lines grown in soil. Top panel, 28-day-old plants; bottom panel, 56-day-old plants. Bars = 200 mm.dThe germination rate of the six lines under normal (control), NaCl or mannitol conditions at day 5 after imbibition.eImages of seeds germinated on either half-strength MS medium (control), medium with 100 mM NaCl or medium with 200 mM mannitol for 7 days. Bars = 50 mm. Asterisks indicated significant differences from the WT, as determined by Student′s t-test (*P< 0.05, **P< 0.01)
Additionally, to evaluate the performance ofZmGPDH1transgenic lines in response to salt or osmotic stresses, the seeds of WT,atgpdhc2,COM and OE lines were germinated on half-strength MS mediums containing 100 mM NaCl or 200 mM mannitol. As shown in Fig.3d and e, seed germination ofatgpdhc2was severely delayed in comparison to the WT, whereas the germination rate ofZmGPDH1OE seeds was much higher than that of other lines. TheZmGPDH1COM seeds displayed stress-sensitive morphologies similarity to the WT, albeit they had a relative higher germination rate (Fig.3d and e).
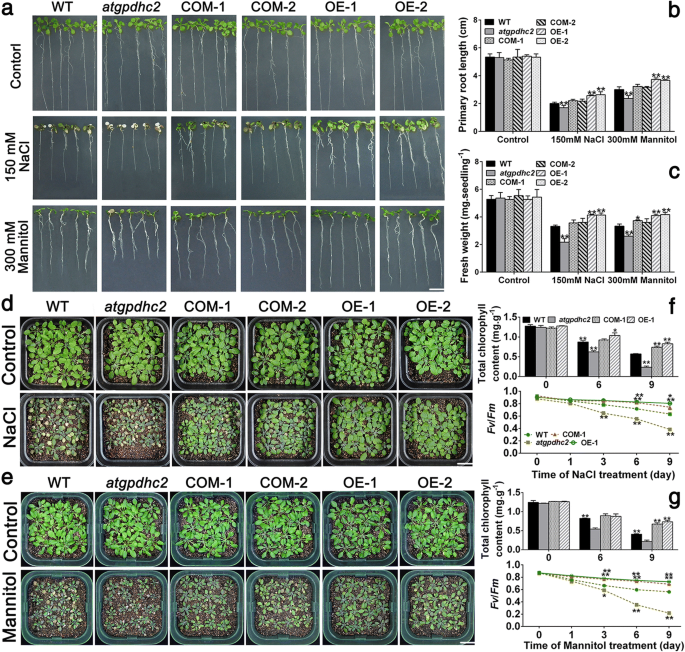
To investigate the impact ofZmGPDH1overexpression on salt/osmotic resistance at early seedling stage, 7-day-old WT,atgpdhc2,COM and OE lines were transplanted to half-strength MS plates supplemented with 300 mM mannitol or 150 mM NaCl for 7 days. As shown in Fig.4a, theatgpdhc2seedlings displayed much severer stress-hypersensitive phenotype than the WT, with a partial leaf bleaching phenomenon. However,ZmGPDH1transformedatgpdhc2(COM-1, COM-2) plants showed wild-type-like phenotype under NaCl and mannitol treatments, indicating that overexpression ofZmGPDH1could rescue salt and osmotic sensitivity ofatgpdhc2mutants. In addition,ZmGPDH1OE plants exhibited an enhanced tolerance to salt/osmotic stress, and a resulting higher fresh weight and root length compared with the WT (Fig.4b and c).
Overexpression ofZmGPDH1enhances the salt- and drought-tolerance in transgenicArabidopsis.aImages of 7-day-old WT,atgpdhc2, complementation (COM-1, COM-2) and overexpression (OE-1, OE-2) plants grown on either half-strength MS plates (control), plates with 300 mM mannitol or 150 mM NaCl for 7 days. Bars = 100 mm.bandcThe primary root length and fresh weight of the six lines after 7 days of treatments. Images of 3-week-old plants irrigated with water (control), 200 mM NaCl (d阿)r 400 mM mannitol (e) every 3 days for 9 days. Bars = 200 mm. The total chlorophyll contents and chlorophyll fluorescence (Fv/Fm阿)f 3-week-old plants irrigated with water (control), 200 mM NaCl (f阿)r 400 mM mannitol (g阿)ver 9 days. Asterisks indicated significant differences (*P< 0.05, **P< 0.01) from the WT, as determined by Student′s t-test
Likewise, when the 3-week-oldArabidopsisplants were subjected to 400 mM mannitol or 200 mM NaCl treatment for 9 days, the growth ofatgpdhc2mutants was strongly inhibited compared with the WT (Fig.4d and e). Conversely, theZmGPDH1OE or COM plants showed obviously improved tolerance to salinity or osmotic stress relative to the other lines (Fig.4d and e). Synchronously, the chlorophyll fluorescence parameter (Fv/Fm) and total chlorophyll content were analyzed inatgpdhc2, COM-1, WT and OE-1 plants. Under standard conditions, chlorophyll content andFv/Fmhad no differences among all four lines (Day 0); however, after exposure to 400 mM mannitol or 200 mM NaCl treatment,atgpdhc2mutant demonstrated a remarkable reduction inFv/Fmand chlorophyll content, whereas theFv/Fmratio and total chlorophyll content in OE-1 and COM-1 were much higher than that in WT plants (Fig.4f and g). These data suggested that overexpression ofZmGPDH1helped to enhance the photochemical efficiency in transgenicArabidopsis.
ZmGPDH1regulates glycerol-3-phosphate and glycerol levels under salt and osmotic stresses
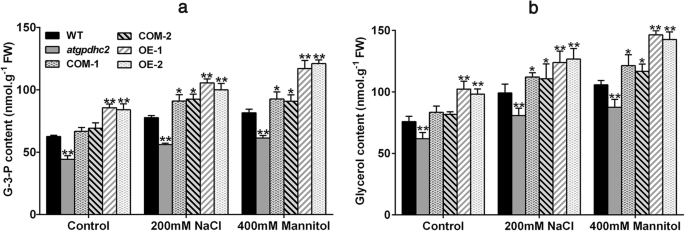
Glycerol is an important compatible solute and the physiological significance of glycerol biosynthesis under salinity or osmotic condition has been reported in many species [4,12,20]。G-3-P is a primary substrate for glycerol synthesis [21]。To characterize the effect ofZmGPDH1on glycerol metabolism, we assayed G-3-P and glycerol levels in 3-week-old WT,atgpdhc2, COM and OEArabidopsislines treated with 200 mM NaCl or 400 mM mannitol for 6 days. Under normal soil conditions, significant reduction in G-3-P and glycerol contents were observed ingpdhc2mutants, while the average contents of G-3-P and glycerol were increased in the OE and COM lines compared with WTArabidopsis(Fig.5a and b). After treatment with NaCl or mannitol, the OE and COM plants accumulated higher level of G-3-P and glycerol than the other lines (Fig.5a and b), indicating that the overexpression ofZmGPDH1could promote the cellular glycerol biosynthesis under high salinity or hyperosmotic stress.
Overexpression ofZmGPDH1increases the glycerol-3-phosphate and glycerol levels under salt and osmotic stresses.aandbThe glycerol-3-phosphate and glycerol contents in 3-week-old WT,atgpdhc2, COM-1, COM-2, OE-1 and OE-2 seedlings irrigated with water (control), 400 mM mannitol and 200 mM NaCl every 3 days for 6 days. Asterisks indicated significant differences from the WT by Student′s t-test (*P< 0.05, **P< 0.01)
ZmGPDH1is essential for redox homeostasis under salt and osmotic stresses
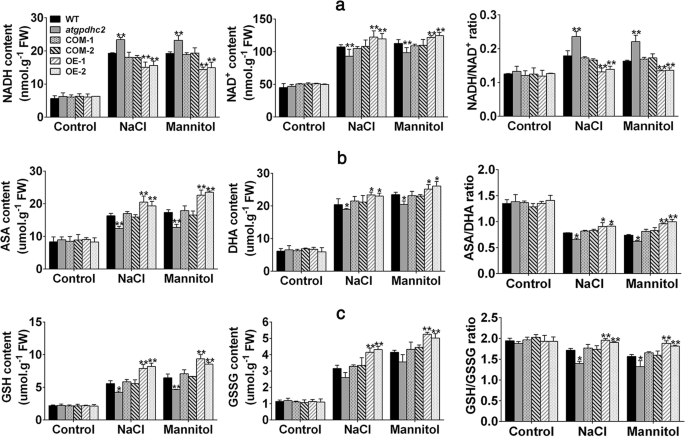
It has been reported that the cytosolic NAD+-GPDH is involved in NADH:NAD+recycling by catalyzing the conversion of DHAP to G-3-P using NADH as a reducing equivalent [8]。To validate if overexpression ofZmGPDH1could affect NADH/NAD+homeostasis upon salt and osmotic stresses, the fluctuation in redox status of NADH was monitored. Under normal growth condition, no significant differences were observed in NADH, NAD+contents and NADH/NAD+ratio among WT,atgpdhc2, COM-1, COM-2, OE-1 and OE-2 lines (Fig.6)。然而,NADH / NAD严重干扰+homeostasis appeared in all the six lines under salt or mannitol treatment, differences could also be seen in the individual NADH or NAD+contents. Although stress treatments elevated the NADH level in each line, theZmGPDH1OE plants accumulated comparatively lower NADH and higher NAD+contents in comparison to WT plants, resulting in a decreased NADH/NAD+ratio (Fig.6a). Nevertheless,atgpdhc2mutants accumulated more NADH and substantially less NAD+content than WT or COM plants, leading to a higher NADH/NAD+ratio. These results illustrated that overexpression ofZmGPDH1might facilitate the oxidation of the excessive reductant (NADH) induced by salt and osmotic stresses, thus increased the NAD+/NADH ratio.
Overexpression ofZmGPDH1maintains the redox homeostasis under salt and osmotic stresses.aNADH contents, NAD+contents, NADH/NAD+ratios;bASA contents, DHA contents, ASA/DHA ratios;cGSH contents, GSSG contents, GSH/GSSG ratio in 3-week-old WT,atgpdhc2, COM-1, COM-2, OE-1 and OE-2 seedlings irrigated with water (control), 400 mM mannitol and 200 mM NaCl every 3 days for 6 days. Asterisks indicated significant differences from the WT by Student′s t-test (*P< 0.05, **P< 0.01)
To further examine whether overexpression ofZmGPDH1could influence the other redox couples, the cellular oxidized and reduced pools of ASA and GSH were also determined. Similarly, the contents of ASA, GSH and their oxidized form DHA, GSSG did not change among the six lines under normal growing conditions (Fig.6b and c). However, when the plants were treated with NaCl or mannitol, theZmGPDH1OE lines maintained relative higher ASA and GSH contents compared with the WT, leading to an even higher ASA/DHA or GSH/GSSG ratio. By contrast, there was a noticeable decline in reduced ascorbate or glutathione as well as the redox ratio (ASA/DHA, GSH/GSSG) inatgpdhc2mutants relative to WT or COM lines (Fig.6b and c). Collectively, these results implied that the founction ofZmGPDH1in salinity and osmotic tolerance could partly attribute to sustaining the cellular redox homeostasis.
ZmGPDH1regulates the ROS level and cell death under salt and osmotic stresses
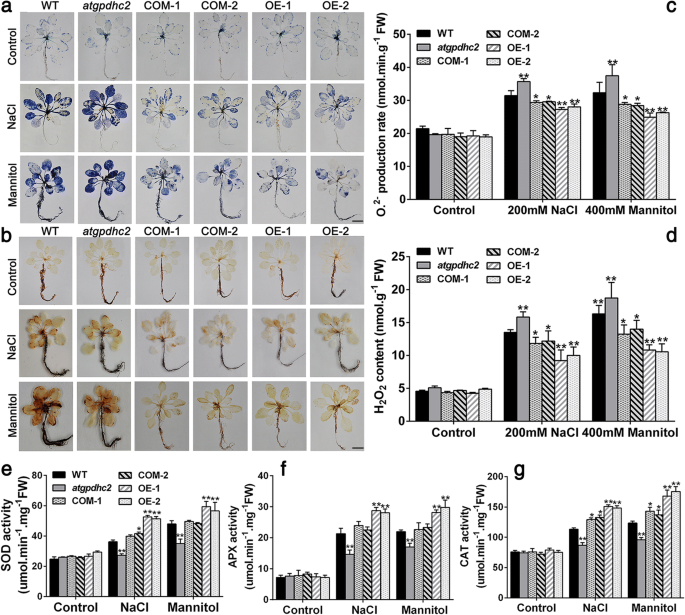
In plant stress reactions, the redox state is highly correlated with the cellular ROS producing and processing [22,23]。Hence, the remarkable changes in redox ratios (NADH/NAD+, ASA/DHA, GSH/GSSG) inZmGPDH1transformed plants promoted us to investigate the ROS level under salt and osmotic stresses. The Fig.7a depicted NBT staining of O2.-, while the Fig.7b illustrated DAB staining of H2O2. In both cases, heavier coloration, reflecting the elevated level of ROS, was detected inatgpdhc2mutants after NaCl or mannitol treatment. On the contrary, the slighter coloration of NBT and DAB staining were observed in both COM and OE plants under identical conditions. Meanwhile, quantitative measurements showed that the contents of H2O2and O2.-in COM and OE plants were markedly lower than that in the WT andatgpdhc2mutants, suggesting the vital role ofZmGPDH1in modulating the cellular ROS accumulation under salt or osmotic stress (Fig.7c and d). The increased ROS production had the potential to trigger the compensatory responses of antioxidant enzymes, therefore, the activities of ROS-scavenging enzymes, including catalase (CAT), ascorbate peroxidase (APX) and superoxide dismutase (SOD) were also determined in this study [23]。As expected, an enhanced activity of these antioxidant enzymes was detected in all the lines under salinity or osmotic condition, while the elevation in OE or COM lines was more prominent than that in the WT (Fig.7e, f and g). In reverse, theatgpdhc2mutant possessed relatively lower antioxidant enzymes activities compared with the WT.
Overexpression ofZmGPDH1enhances the ROS scavenging capacity under salt and osmotic stresses. Photographs showing representative (a) NBT and (b) 3,3-diaminobenzidine (DAB) staining of 3-week-old WT,atgpdhc2, COM-1, COM-2, OE-1 and OE-2 plants treated with water (control), 200 mM NaCl and 400 mM mannitol for 2 h. Bars = 50 mm. O2.-(c) and H2O2(d) levels in the six lines after 6 days of water (control), 200 mM NaCl or 400 mM mannitol treatment. Enzyme activity of(e)superoxide dismutase (SOD), (f) catalase (CAT) and (g) ascorbate peroxidase (APX) in the six lines treated as above. Asterisks indicated significant differences from the WT by Student′s t-test (*P< 0.05, **P< 0.01)
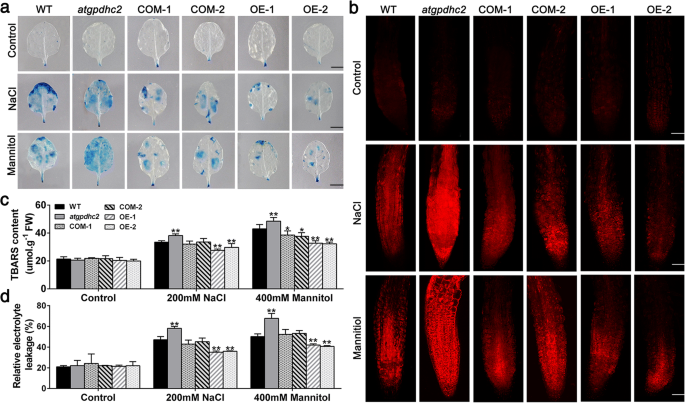
Additionally, the oxidative stress mediated cell death was also detected by Evan′s blue and propidium iodide (PI) staining [24]。As shown in Fig.8a和b,温和的强度Evan的蓝色和π圣aining was observed in all the six lines under the normal conditions. However, the cell death was strongly stimulated inatgpdhc2mutants, moderately stimulated in WT or COM and mildly stimulated in OE line after treatment with NaCl or mannitol (Fig.8aand b). The TBARS and electrolyte leakage levels, as the measures of oxidative damages in cell membranes, were significantly lower in OE line than that in the other lines under salt and osmotic stresses (Fig.8c and d). Clearly, these findings indicated that overexpression ofZmGPDH1alleviated the cellular ROS accumulation by improving antioxidant defense and consequently minimize the cell death as well as membrane lipid peroxidation under salt and osmotic stresses.
Overexpression ofZmGPDH1alleviates the stress-induced membrane injury and cell death.aThe Evan′s Blue staining in 3-week-old WT,atgpdhc2, COM-1, COM-2, OE-1 and OE-2 lines irrigated with water (control), 400 mM mannitol and 200 mM NaCl for 2 h. Bars = 50 mm.bThe propidium iodide (PI) fluorescence staining in root tips of 7-day-old plants treated on either half-strength MS plates (control), plates with 300 mM mannitol or 150 mM NaCl for 12 h. The images were obtained by cofocal microscope. Bars = 100 μm. TBARS contents (c) and the relative electrolyte leakage (d阿)f the six lines after 6 days of water (control), 200 mM NaCl or 400 mM mannitol treatments. Asterisks indicated significant differences from the WT by Student′s t-test (*P< 0.05, **P< 0.01)
ZmGPDH1is involved in stress-induced stomatal closure
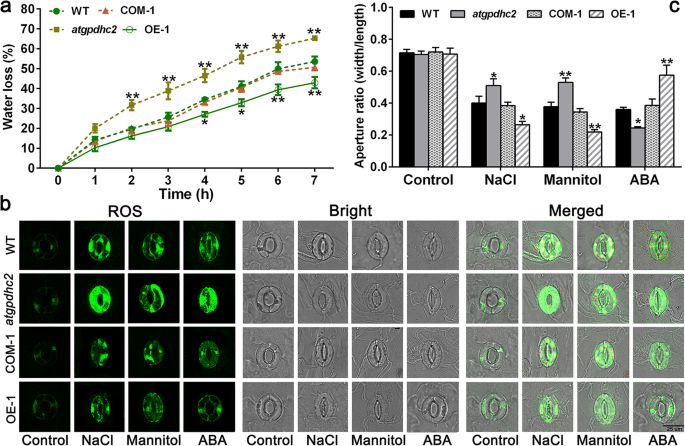
The water loss measurement revealed that overexpression ofZmGPDH1remarkably enhanced the transgenic plants resistance to water deficit, as reflected by a lower WLR (Fig.9a). Since water loss mainly depends on the stomatal regulation, we further valuated whetherZmGPDH1was involved in the modulation of the stomatal closure under stress treatments. There was no significant difference in guard cells size or stomatal aperture ratio among WT,atgpahc2, COM-1 and OE-1 lines prior to stress treatment (Fig.9b). By contrast, the NaCl or mannitol treatment markedly induced the stomatal closure in all the lines; nevertheless, the stomatal aperture ratio was dramatically lower in OE-1 plants and higher inatgpdhc2mutants, compared with WT plants. In addition, the stomatal aperture in COM-1 plants was generally similar with that in the WT (Fig.9c). This implied thatZmGPDH1played an essential role in regulating the stomatal response to salt/osmotic stress.
Overexpression ofZmGPDH1promotes the stomatal closure under salt and osmotic stresses.aThe analysis of water loss rate (WLR) of the WT,atgpdhc2, COM-1 and OE-1.b气孔关闭化验,远轴的表皮of rosette leaves were incubated in the light for 1 h to induce the stomatal opening and then treated with water (control), 300 mM mannitol, 150 mM NaCl and 20 μM ABA for 3 h. Bars = 25 μm.cStomatal aperture was investigated by measuring the length and width of guard cells. Asterisks indicated significant differences from the WT, as determined by Student′s t-test (*P< 0.05, **P< 0.01)
The phytohormone ABA has the ability to induce stomatal closure [25]; however, overexpression ofZmGPDH1led to ABA insensitivity in stomatal movement (Fig.9b). In the presence of ABA, the stomatal closure was significantly triggered inatgpdhc2mutants, moderately triggered in WT or COM and mildly triggered in OE plants, illustrating thatZmGPDH1-mediated stomatal closure was independent of ABA (Fig.9c). In addition, the H2O2production in the guard cells was also determined by H2DCF-DA staining. As shown in Fig.9b, the H2O2accumulation was less in OE plants and more inatgpdhc2mutant compared with that in the WT, suggesting that overexpression ofZmGPDH1contributed to sustain the ROS levels during stomatal movements.
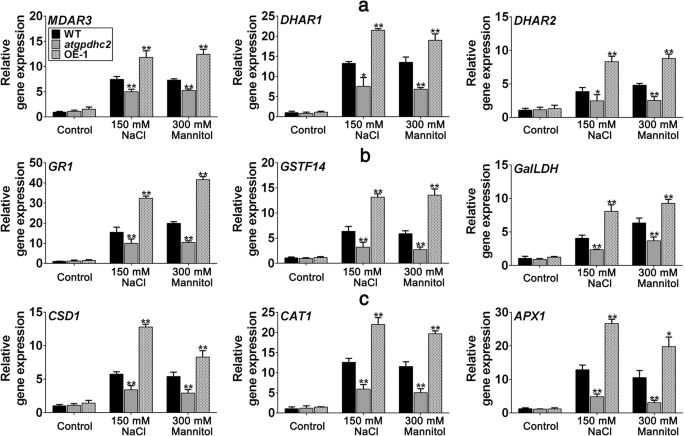
Effects ofZmGPDH1on expression of genes involved in redox homeostasis and ROS-scavenging system
Next, to elucidate the impact ofZmGPDH1on molecular basis of salinity or osmotic stress response of cellular redox and ROS homeostasis, we detected the transcripts of a number of key genes involved in (1) ASA and GSH metabolism: cytosolic monodehydro-ascorbate reductase (MDAR3), cytosolic glutathione reductase (GR1), cytosolic dehydroascorbate reductase (DHAR1,DHAR2), cytosolic glutathione transferase (GSTF14) and cytosolic L-galactose dehydrogenase (GalLDH) [26,27,28] (2) ROS-scavenging system: cytosolic copper/zinc superoxide dismutase (CSD1), cytosolic catalase (CAT1) and cytoplasmic ascorbate peroxidase (APX1) [29,30]。As shown in Fig.10, the transcripts of all genes tested inatgpdhc2and OE-1 lines were generally similar to WT under normal conditions. Upon salinity or osmotic treatment, the transcripts of genes participating in ASA-GSH redox cycle (MDAR3,GR1,DHAR1,DHAR2w)ere decreased inatgpdhc2mutant but elevated inZmGPDH1OE lines compared with the WT (Fig.10a). Similarly, the transcripts of genes related to the biosynthesis of ASA and GSH (GSTF14,GalLDHw)ere also significantly increased in OE plants, despite which is not the case inatgpdhc2mutants. In addition, the transcripts ofCSD1,CAT1andAPX1were highly stimulated by NaCl or mannitol treatment, and were higher inZmGPDH1OE line but lower in theatgpdhc2compare to the WT, indicating that overexpression ofZmGPDH1up-regulated the expression of the cytosolic antioxidant-related genes under both salt and osmotic stresses (Fig.10b). These data also supported the finding that antioxidant activities of APX, SOD and CAT increased in theZmGPDH1OE transgenic plants. Taken together, these results indicated thatZmGPDH1might function in the regulation of cellular redox and ROS homeostasis to prevent the oxidative damage caused by salt or osmotic stress.
Overexpression ofZmGPDH1increases the transcripts of genes involved in cellular redox and ROS homeostasis.aandbTranscripts of genes involved in redox homeostasis (MDAR3,DHAR1,DHAR2,GR1,GSTF14andGaILDH).cTranscripts of genes involved in ROS-scavenging system (CSD1,CAT1andAPX1). The roots of 3-week-old WT,atgpdhc2, and OE lines were submerged with water (control), 300 mM mannitol or 150 mM NaCl solution for 24 h, respectively. The expression of each gene in the WT treated with water was used to normalize its transcripts in different lines under different conditions. Asterisks indicated significant differences from the WT by Student′s t-test (*P< 0.05; **P< 0.01)
Discussion
Maize (Zea maysL.) is an important cereal crops as well as a major source of biofuel, industrial material and animal feed [31]。Although a great deal of research has indicated that glycerol-3-phosphate dehydrogenase (GPDH) plays a pivotal role in plant growth and stress adaptions [3,17,21], little is currently known about its functions in field crops including maize. In this study, we isolated aGPDHgene encoding NAD+-dependent GPDH from maize. Similar to other typical NAD+-dependent GPDH [14,16,19], ZmGPDH1 protein contained the necessary and specific protein domains (PF07479, PF01210). The conserved GAGAWG motif was found at residues 44–50 of the protein sequence ofZmGPDH1(Additional file1: Figure S1), which was similar to the previously reported NAD+-dependent GPDH isoforms with an analogous NAD+-binding fragments corresponding to GXGXXG [8,10,32]。
Enzymatic assay of recombinant ZmGPDH1 proteins expressed inEscherichia coliRosetta strain (DE3) (Fig.1e and f) showed the purified ZmGPDH1 protein had substrate affinity (KmDHAPof 2.75 mM) (Fig.1e), which compared well with the kinetic parameters previously reported for other GPDH enzymes [10,33,34]。稳定或瞬时表达ssion of a green fluorescent protein (GFP)-taggedZmGPDH1inArabidopsisor wild-type rice were conducted, and both evidenced that ZmGPDH1 proteins were specially targeted to cytosol (Additional file2: Figure S2 and Fig.1a). The earlier identifiedArabidopsisGPDH proteins (AtGPDHc1 and AtGPDHc2) were predicted to be cytosol-located GPDs owing to the absence of apparent transmembrane regions and subcellular targeting sequences, however, a clear experimental evidence was lacking [5,8]。
In succession, the physiological functions of the cytosolicZmGPDH1gene in mediating salinity/osmotic adaption were investigated in this study. The transcript abundance ofZmGPDH1was markedly increased under NaCl and mannitol treatments (Fig.2n and o). On the other hand, the transgenicArabidopsisharboring theZmGPDH1promoter fused to aGUSreporter gene also showed relatively higher GUS activity under NaCl or mannitol condition (Fig.2p), indicating thatZmGPDH1gene was regulated at the transcription level in response to salt or osmotic stress, which was consistent with the expression patterns of otherGPDHgenes fromA.thaliana(AtGPDHc1,AtGPDHm1),C. reinhardtii(CrGPDH2,CrGPDH3),D. salina(DsGPDH2,G3PDH) andD. viridis (DvGPDH1,DvGPDH2) [6,8,9,13,19]。Furthermore, overexpression ofZmGPDH1strongly enhanced the tolerance ofArabidopsis(WT) to salt/osmotic stress and rescued the salt/osmotic sensitivity ofatgpdhc2mutant, as reflected by a pronounced elevation in germination rate, fresh weight, root length, biomass, chlorophyll content andFv/Fmratio under salinity or osmotic conditions (Figs.3and4). Similar results have been reported earlier in other species: overexpression of an oyster mushroomGPDHgene (PsGPD) increased the salt tolerance in transgenic potatoes and rice; and overexpression of a black yeastGPDHgene (HwGPD1B) also enhanced NaCl tolerance ofSaccharomyces cerevisiae gpd1mutant [17,35]。
We also found thatZmGPDH1gene was required for glycerol generation. Glycerol is an important osmo-protectant and its accumulation can compensate for differences between intracellular and extracellular water potentials under hyperosmotic environment [6,12,13]。In our study, overexpression ofZmGPDH1markedly increased the levels of G-3-P and glycerol, demonstrated thatZmGPDH1played essential roles in plant adaptation to hypersaline or hyperosmotic shock by contributing to the glycerol biosynthesis (Fig.5). Likewise, of the five GPDH enzymes inChlamydomonas reinhardtii,CrGPDH2andCrGPDH3were shown to be necessary for osmotic-induced glycerol production [36]。Also, loss ofAtGPDHc1gene encoding a cytosol-localized GPDH ofArabidopsiscaused the hypersensitivity to salt stress due to the severe impairment in provision of glycerol [8]。
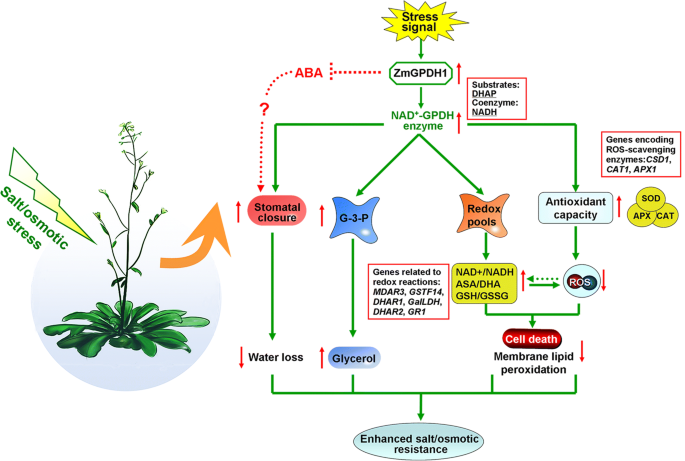
Most notably, cytosolic GPDHs are reported to participate in the mitochondrial G-3-P shuttle system, which functions as a pivotal route to keep the cellular redox status inArabidopsis[8]。In this study,ZmGPDH1overexpressionArabidopsisshowed significantly decreased NADH level accompanied by an increased NAD+accumulation during salt/osmotic stress (Fig.6a). As a consequence, a noticeable reduction in cellular NADH/NAD+比中检测出OE线,建议ZmGPDH1involved in the regulating of NADH/NAD+redox homeostasis by consuming the excessive redundant NADH and regenerating NAD+under salt or osmotic stress. In addition, an apparent increase in ASA and GSH contents as well as their redox pool were observed inZmGPDH1overexpressionArabidopsiscompared with that in WT plants (Fig.6b and c), indicated that overexpression ofZmGPDH1also affected the redox states of ASA and GSH, apart from a decreased cellular NADH/NAD+ratio. It is known that GR, MDHAR and DHAR are responsible for the regeneration of ASA and GSH in ASA-GSH redox cycle [37]。GalLDH and GST are proved to be essential enzymes involved in the biosynthesis of ASA and GSH, respectively [28]。In the present study, the increased expression of the marker genes corresponding to the above mentioned enzymes in theZmGPDH1-OE plants implied thatZmGPDH1might exert effects on the ASA/GSH redox cycles as well, and play a critical role in optimizing the cellular redox homeostasis of NADH/NAD+, ASA/DHA and GSH/GSSG under salt and osmotic stresses, as speculated in Fig.11. Overexpression ofZmGPDH1also caused a reduction in ROS level, including the guard cell ROS, as exhibited by the slight H2DCF-DA staining under stress treatments (Fig.7and Fig.9). Besides, the level of lipid peroxidation and cell death inZmGPDH1OE lines was much lower than that of the other plants (Fig.8), illustrating that overexpression ofZmGPDH1availably protected cell from oxidative damage and maintained the membrane integrity under salt or osmotic stress. It has also been reported that the soluble redox couples seem to assume a dual role with respect to ROS [28]。On one hand, the excessive NADH can be involved in, or related to ROS-generation metabolic pathway by triggering the over-reduction of molecular oxygen O2[38]。In contrast, the reductive detoxification of ROS also heavily depends on the NADH oxidation, as signified by the increased NAD+/NADH ratio, which helps to enhance the oxidant scavenging capacity [23,28]。Hence, it appeared thatZmGPDH1might participate in the regulation of ROS metabolism by manipulating the cellular NADH/NAD+ratio (Fig.11). On the other hand, the antioxidant system has been proved to be dramatically evoked to eliminate excess ROS under abiotic stress [39]。In agreement with this, we found that the activities and transcripts of ROS-scavenging enzymes (CSD1,CAT1, andAPX1w)ere strongly stimulated by salinity or mannitol in OE or COM plants (Fig.7and Fig.10). In summary, the overexpression ofZmGPDH1resulted in higher expression of genes encoding key enzymes of cytosolic ROS scavenging system involving the SOD/CAT/ascorbate/ glutathione cycle, which led to lower ROS accumulation and higher ROS detoxification capacity, and hence stronger stress tolerance (Fig.11).
Model of the involvement ofZmGPDH1in salt/osmotic stress responses. The stress signals triggered the expression ofZmGPDH1and increased the NAD+-GPDH enzyme activity, which was required for two biological processes: glycerol biosynthesis by affecting the G-3-P provision and NADH/NAD+homeostasis by disposing of extra reducing power. The elevated expression of genes involved in ROS and redox homeostasis resulted in improved ROS scavenging capacity and a series of positive physiological changes. In addition, the boosted accumulation of G-3-P/glycerol and optimized stomatal movement/water loss were also in concurrence, and collectively contributed to an enhanced salt/osmotic resistance. Red arrows indicated the tendency of changes
Additionally, our results showed thatZmGPDH1-OE plants exhibited greater stomatal closure compared with other lines, indicating thatZmGPDH1played a key role in manipulating the stomatal closure under salt or osmotic stress (Fig.9). However, the ABA-induced stomatal closing was impaired inZmGPDH1-OE lines but significantly induced inatgpdhc2mutant, suggesting that theZmGPDH1-mediated stomatal closure might be independent of ABA. Meanwhile, we found that overexpression ofZmGPDH1reduced the plant sensitivity to ABA at seed germination and early seedling developmental stages (Additional file5: Figure S5), which was also observed previously onAtGPDHc1mutants [8]。Further studies will be needed to interpret the interaction ofZmGPDH1and ABA signaling pathway.
Conclusions
We reported the characterization of a cytosolic NAD+-dependent GPDH gene from maize,ZmGPDH1, which had profound effects on salt/osmotic tolerance by regulating the glycerol accumulation, cellular redox homeostasis, ROS-scavenging system as well as stomatal movement (Fig.11).
Methods
Plant materials and growth conditions
The maize inbred line accession He-344 (provided by Heilongjiang Academy of Agricultural Sciences, Harbin, China) was used as the plant material in this experiment and normally planted in a growth chamber under controlled photoperiod and temperature (12 h light/12 h dark, 23 ± 2 °C), with a photon flux density of 1000 μmol m− 2 s− 1.
The seeds of T-DNA insertion mutants ofAtGPDHc2(TAIR: At3G07690), namelyatgpdhc2(SALK_033040) were donated by Dr. Pradeep Kachroo (University of Kentucky, USA). The homozygous lines ofatgpdhc2mutant were identified by PCR and reverse transcription PCR (RT-PCR) analysis, and the primers were shown in Additional file6: Table S1. The plants ofArabidopsis thaliana(ecotype Col) andatgpdhc2mutant (ecotype Col) were grown in a growth chamber under controlled photoperiod and temperature (16 h light/8 h dark, 21 ± 2 °C), with a photon flux density of 100 μmol m− 2 s− 1.
Plasmid construction and plant transformation
The full length coding region ofZmGPDH1(1377 bp) was cloned from cultivated maize by the gene specific primers (Additional file6: Table S1). The PCR product was purified and inserted into theXbaI andSalI sites of the pBI121-GFP vector under control of the CaMV35S promoter. For complementation and over-expression assays,Agrobacterium tumefaciensstrain EHA105 carrying the constructpBI121-ZmGPDH1::GFPwas used to transformArabidopsiswild-type oratgpdhc2. T3 homozygous transgenicArabidopsiswere screened by RT-PCR.
Protein subcellular localization and GUS activity assay
To verify the subcellular localization ofZmGPDH1, the mesophyll protoplasts were isolated from T3 homozygous transgenicArabidopsisharboringpBI121-ZmGPDH1::GFPorpBI121-GFP (control) plasmid, and the subcellular localization of GFP expression was visualized by confocal laser-scanning microscope (Leica, German). The positive control (empty vector) or fusion proteins were also temporarily expressed in rice mesophyll protoplasts according to the methods described previously [40]。For co-localization studies, a far-red fluorescent protein mkate was used as the cytosol marker. To study the promoter activity, a 1642-bp genomic region upstream of the translation initiation codon ofZmGPDH1gene was cloned into pBI121-GUS atHindIII andXbaI sites (primers see Additional file1: Table S1). The constitutiveproZmGPDH1::GUStransformed WT plants were also generated and T3 homozygous transgenic lines were used for GUS staining according to the reported protocol [41]。The images were visualized by stereo microscope (Olympus, Japan).
Recombinant ZmGPDH1 protein expression, purification and western blot
The coding region ofZmGPDH1with theNcoI andXhoI sites was amplified by PCR and then inserted into the pET32a (+) vector containing 6 × His tag. ThepET32a-ZmGPDH1plasmid was transformed into theEscherichia coli(E. coli) Rosetta strain and the expression ofZmGPDH1was induced with 1 mM IPTG to generate the putative recombinants. Then the His-tagged ZmGPDH1 proteins were extracted and purified under native conditions using Ni-NTA nickel columns (Sigma), and the purified proteins were detected by 12% SDS-PAGE as well as Western blot using 6 × His Tag Antibody as probe.
Phenotypic analyses of theZmGPDH1transgenicArabidopsisunder salt or osmotic treatment
For germination analysis, seeds of WT,atgpdhc2, OE and COM lines were plated on half-strength MS plates containing 200 mM mannitol or 100 mM NaCl for 8 days. Germination rates were counted at day 5 after sowing and seed germination was defined as the appearance of visible radicle. To investigate the effects of salinity and osmotic stresses on root length and fresh weight, 7-days-old seedlings of WT,atgpdhc2mutant, COM and OE lines were transferred into half-strength MS plates supplemented with 150 mM NaCl or 300 mM mannitol. The root length and fresh weights of stress-treated seedlings were determined after 7 day of treatment.
For the stress tolerance test at the adult stage, 3-week-oldArabidopsisplants were irrigated with 200 mM NaCl or 400 mM mannitol solution every 3 days for a total of 9 days. Rosette leaf samples were collected at day 6 of treatments to measure the changes of various physiological and biochemical parameters. All experiments were replicated at least three times with 80–100 plants per treatment. Photographs taken from one representative experiment are shown. Total chlorophyll (chlorophyll a + b) was determined according to the method as previously described [42] and the fresh young leaf was extracted in 80% (v/v) acetone extract. Photochemical efficiency (Fv/Fmw)as examined by using a pulse-modulated fluorometer (FMS2, Hansatech, UK) [43]。
To investigate the water loss rate (WLR), the rosette leaves from 4-week-oldArabidopsiswere weighed at specific time points. The decrease in fresh weight was used to calculate WLR. For stomatal closure assays, the strips abaxial epidermis ofArabidopsisleaves were immerged in buffer (10 mM MES/KOH, pH 6.1, 10 mM KCl, 50 μM CaCl2) under light for 1 h to induce the stomatal opening and then treated with 300 mM mannitol, 150 mM NaCl and 20 μM ABA for 3 h. The conformation of stomatal aperture were photographed by confocal microscope and processed with ImageJ software. The experiments were replicated at least three times with 40–50 cells per treatment.
Analysis of GPDH activity, G-3-P and glycerol levels
G-3-P and glycerol contents were measured as previously described with slight modifications [44]。GPDH活动检查了关于the reduction of DHAP by NADH. The total reaction volume of the assay was 1 mL containing 100 mM HEPES buffer, pH 6.9, 4 mM DHAP, 0.2 mM NADH and an appropriate amount of enzyme [8]。The absorbance changes at 340 nm were monitored using an ultraviolet spectrophotometer (U3900, Hitachi High-Technologies, Japan).
Analysis of cellular redox and ROS homeostasis
The reduced pyridine nucleotides (NADH) content, oxidized pyridine nucleotides (NAD+) content and NADH/NAD+ratio were assayed with an enzymatic cycling procedure [45]。The ascorbate (ASA) content, dehydroascorbate (DHA) content and ASA/DHA ratio were measured following the reported protocols [46]。The glutathione (GSH) content, oxidized glutathione (GSSG) content and GSH/GSSG ratio were assayed as described [47]。
For ROS accumulation analysis, the staining of nitroblue tetrazolium (NBT) and 3,3- diaminobenzidine (DAB) of stress-treated seedlings were performed following the reported protocol [48]。For hydrogen peroxide (H2O2) staining in the guard cells, prepared epidermal peels with NaCl, mannitol or ABA treatment were stained with 2,7-dichlorofluorescin diacetate (H2DCF-DA) for 10 min [49]。Cell death caused by salt or osmotic stress was also estimated by Evan′s blue and PI staining as described [24]。The assays of H2O2和过氧化物(O2.-w)ere conducted by spectrophotometry as previously described [50,51]。The lipid peroxidation was measured with reference to the thiobarbituric acid-reactive substances (TBARS) content [52]。Electrolyte leakage (EL) was assessed as described [53]。
To monitor the antioxidant enzyme activities, leaf tissues (0.5 g) were ground in ice bath with 10 mL extraction buffer (K2HPO4-KH2PO4, pH 7.0, 1.5 mM EDTA, 1% PVP, 0.5 mM ASC), and then the homogenate was centrifuged at 12000 rpm for 20 min at 4 °C. The supernatant was used for the determination of enzymes activities. The activities of catalase (CAT), ascorbate peroxidase (APX) and superoxide dismutase (SOD) were determined as described [54,55], with slight modifications.
Quantitative real-time RT-PCR analysis
To analyze the expression ofZmGPDH1under osmotic and salt stresses, 3-week-old maize seedlings were treated with 1/2 Hoagland solution containing 400 mM mannitol and 200 mM NaCl solutions for 0, 1, 3, 6, 12 and 24 h, and the roots were sampled to analyze the transcripts ofZmGPDH1. The untreated maize samples from the same time point were used as the controls.ZmGAPDHandZmACTINgenes served as internal reference in each assay. To examine the tissue-specific expression ofZmGPDH1从玫瑰叶子,总RNA提取(RL), flower buds (FLB), roots (RT), flowers (FL), siliques (SL), stems (ST) and cauline leaves (CL) inproZmGPDH1::GUStransgenic plants. To analyze target genes expression induced by osmotic and salt stresses, 3-week-old WT,atgpdhc2, and OE lines were treated with water (control), 300 mM mannitol or 150 mM NaCl solution. Total RNA was extracted from rosette leaf samples at 24 h after treatments. The expression of target genes in WT plants under control environment was used as a calibrator.ACTIN2andUBQ7genes were used as internal reference [56]。The primers used for transcriptional analysis were shown in Additional file6: Table S1.
Statistical analysis
Data are presented as Mean ± SD. The Student’s t-test was used to determine the significance levels using SPSS 21.0 software throughout this study. AP-value of < 0.05 was considered statistically significant.
Abbreviations
- ABA:
-
Abscisic acid
- APX:
-
Ascorbate peroxidase
- ASA:
-
Ascorbate
- CAT:
-
Catalase
- CL:
-
Cauline leaves
- DAB:
-
3,3-diaminobenzidine
- DHA:
-
Dehydroascorbate
- DHAP:
-
Dihydroxyacetone phosphate
- EL:
-
Electrolyte leakage
- FL:
-
Flowers
- FLB:
-
Flower buds
- G-3-P:
-
Glycerol-3-phosphate
- GFP:
-
Green fluorescent protein
- GK:
-
Glycerol kinase
- GPDH:
-
Glycerol-3-phosphate dehydrogenase
- GSH:
-
Glutathione
- GSSG:
-
Oxidized glutathione
- H2DCF-DA:
-
2,7-dichlorofluorescin diacetate
- H2O2:
-
Hydrogen peroxide
- NAD+:
-
Oxidized pyridine nucleotides
- NADH:
-
Reduced pyridine nucleotides
- NBT:
-
Nitroblue tetrazolium
- O2.-:
-
Superoxide
- PI:
-
Propidium iodide
- qRT-PCR:
-
quantitative real-time PCR
- RL:
-
Rosette leaves
- RT:
-
Roots
- RT-PCR:
-
reverse transcription PCR
- SL:
-
Siliques
- SOD:
-
Superoxide dismutase
- ST:
-
Stems
- TBARS:
-
Thiobarbituric acid-reactive substances
- WLR:
-
Water loss rate
- WT:
-
Wild-type
References
- 1.
Haslam RP, Sayanova O, Kim HJ, Cahoon EB, Napier JA. Synthetic redesign of plant lipid metabolism. Plant J. 2016;87(1):76–86.
- 2.
Klein M, Swinnen S, Thevelein JM, Nevoigt E. Glycerol metabolism and transport in yeast and fungi: established knowledge and ambiguities. Environ Microbiol. 2017;19(3):878–93.
- 3.
Oren A. Glycerol metabolism in hypersaline environments. Environ Microbiol. 2017;19(3):851–63.
- 4.
Eastmond PJ. Glycerol-insensitiveArabidopsismutants:gli1seedlings lack glycerol kinase, accumulate glycerol and are more resistant to abiotic stress. Plant J. 2004;37(4):617–25.
- 5.
Chanda B, Xia Y, Mandal MK, Yu K, Sekine KT, Gao QM, Selote D, Hu Y, Stromberg A, Navarre D, Kachroo A, Kachroo P. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat Genet. 2011;43(5):421–7.
- 6.
Chen H, Lu Y, Jiang J-G. Comparative analysis on the key enzymes of the glycerol cycle metabolic pathway inDunaliella salinaunder osmotic stresses. PLoS One. 2012;7(6):e37578.
- 7.
Guadalupe-Medina V, Metz B, Oud B, van Der Graaf CM, Mans R, Pronk JT, van Maris AJA. Evolutionary engineering of a glycerol-3-phosphate dehydrogenase-negative, acetate-reducingSaccharomyces cerevisiaestrainenables anaerobic growth at high glucose concentrations. Microb Biotechnol. 2014;7(1):44–53.
- 8.
Shen W, Wei Y, Dauk M, Tan Y, Taylor DC, Selvaraj G, Zou J. Involvement of a glycerol-3- phosphate dehydrogenase in modulating the NADH/NAD+ratio provides evidence of a mitochondrial glycerol-3-phosphate shuttle inArabidopsis. Plant Cell. 2006;18(2):422–41.
- 9.
Shen W, Wei Y, Dauk M, Zheng Z, Zou J. Identification of a mitochondrial glycerol-3-phosphate dehydrogenase fromArabidopsis thaliana: evidence for a mitochondrial glycerol-3-phosphate shuttle in plants. FEBS Lett. 2003;536(1–3):92–6.
- 10.
Wei Y, Periappuram C, Datla R, Selvaraj G, Zou J. Molecular and biochemical characterizations of a plastidic glycerol-3-phosphate dehydrogenase fromArabidopsis. Plant Physiol Biochem. 2001;39(10):841–8.
- 11.
Singh V, Singh PK, Siddiqui A, Singh S, Banday ZZ, Nandi AK. Over-expression ofArabidopsis thaliana SFD1/GLY1, the gene encoding plastid localized glycerol-3-phosphate dehydrogenase, increases plastidic lipid content in transgenic rice plants. J Plant Res. 2016;129(2):285–93.
- 12.
Petrovic U, Gunde CN, Plemenitas A. Cellular responses to environmental salinity in the halophilic black yeastHortaea werneckii. Mol Microbiol. 2010;45(3):665–72.
- 13.
He Y, Meng X, Fan Q, Sun X, Xu Z, Song R. Cloning and characterization of two novel chloroplastic glycerol-3-phosphate dehydrogenases fromDunaliella viridis. Plant Mol Biol. 2009;71(1–2):193–205.
- 14.
Cai M, He L-H, Yu T-Y. Molecular clone and expression of a NAD+-dependent glycerol-3- phosphate dehydrogenase isozyme gene from the halotolerant algaDunaliella salina. PLoS One. 2013;8(4):e62287.
- 15.
他问,乔D,白L,张问,杨W,李问,曹Y。Cloning and characterization of a plastidic glycerol-3-phosphate dehydrogenase cDNA fromDunaliella salina. J Plant Physiol. 2007;164(2):214–20.
- 16.
Morales-Sánchez D, Kim Y, Terng EL, Peterson L, Cerutti H. A multidomain enzyme, with glycerol-3-phosphate dehydrogenase and phosphatase activities, is involved in a chloroplastic pathway for glycerol synthesis inChlamydomonas reinhardtii. Plant J. 2017;90(6):1079–92.
- 17.
Cho JI, Lim HM, Siddiqui ZS, Park SH, Kim AR, Kwon TR, Lee SK, Park SC, Jeong MJ, Lee GS. Over-expression ofPsGPD, a mushroom glyceraldehyde-3-phosphate dehydrogenase gene, enhances salt tolerance in rice plants. Biotechnol Lett. 2014;36(8):1641–8.
- 18.
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Phys. 2000;51(1):463–99.
- 19.
Driver T, Trivedi DK, McIntosh OA, Dean AP, Goodacre R, Pittman JK. Two glycerol-3-phosphate dehydrogenases fromChlamydomonashave distinct roles in lipid metabolism. Plant Physiol. 2017;174(4):2083–97.
- 20.
Bahieldin A, Sabir JSM, Ramadan A, Alzohairy AM, Younis RA, Shokry AM, Gadalla NO, Edris S, Hassan SM, Al-Kordy MA, Kamal KBH, Rabah S, Abuzinadah OA, El-Domyati FM. Control of glycerol biosynthesis under high salt stress in Arabidopsis. Funct Plant Biol. 2014;41(1):87.
- 21.
Yao Y, Lu Y, Peng KT, Huang T, Niu YF, Xie WH, Yang WD, Liu JS, Li HY. Glycerol and neutral lipid production in the oleaginous marine diatomPhaeodactylum tricornutumpromoted by overexpression of glycerol-3-phosphate dehydrogenase. Biotechnol Biofuels. 2014;7(1):110.
- 22.
Hossain MS, Dietz KJ. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front Plant Sci. 2016;7:548.
- 23.
Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17(7):1866–75.
- 24.
Kim M, Ahn JW, Jin UH, Choi D, Paek KH, Pai HS. Activation of the programmed cell death pathway by inhibition of proteasome function in plants. J Biol Chem. 2003;278(21):19406–15.
- 25.
He F, Wang HL, Li HG, Su Y, Li S, Yang Y, Feng CH, Yin W, Xia X. PeCHYR1, a ubiquitin E3 ligase fromPopulus euphratica, enhances drought tolerance via ABA-induced stomatal closure by ROS production inPopulus. Plant Biotechnol J. 2018;16:1514–28.
- 26.
Yin L, Ji M, Tanaka K, Wang S, Zhang M, Deng X, Zhang S. High level of reduced glutathione contributes to detoxification of lipid peroxide-derived reactive carbonyl species in transgenicArabidopsisoverexpressing glutathione reductase under aluminum stress. Physiol Plantarum. 2017;161(2):211–23.
- 27.
Ding ZJ, Yan JY, Xu XY, Yu DQ, Li GX, Zhang SQ, Zheng SJ. Transcription factorWRKY46regulates osmotic stress responses and stomatal movement independently inArabidopsis. Plant J. 2014;79(1):13–27.
- 28.
Noctor G. Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ. 2006;29(3):409–25.
- 29.
Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R. Ascorbate peroxidase 1 plays a key role in the response ofArabidopsis thalianato stress combination. J Biol Chem. 2008;283(49):34197–203.
- 30.
Vishwakarma A, Tetali SD, Selinski J, Scheibe R, Padmasree K. Importance of the alternative oxidase (AOX) pathway in regulating cellular redox and ROS homeostasis to optimize photosynthesis during restriction of the cytochrome oxidase pathway inArabidopsis thaliana. Ann Bot-london. 2015;116(4):555–69.
- 31.
Lobell DB, Hammer GL, McLean G, Messina C, Roberts MJ, Schlenker W. The critical role of extreme heat for maize production in the United States. Nat Clim Chang. 2013;3(5):497–501.
- 32.
Herrera-Valencia VA, Macario-González LA, Casais-Molina ML, Beltran-Aguilar AG, Peraza-Echeverría S. In silico cloning and characterization of the glycerol-3-phosphate dehydrogenase (GPDH) gene family in the green microalgaChlamydomonas reinhardtii. Curr Microbiol. 2012;64(5):477–85.
- 33.
Ansell R, Granath K, Hohmann S, Thevelein JM, Adler L. The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded byGPD1andGPD2have distinct roles in osmoadaptation and redox regulation. EMBO J. 1997;16(9):2179–87.
- 34.
Kirsch T, Gerber DW, Byerrum RU, Tolbert NE. Plant dihydroxyacetone phosphate reductases: purification, characterization, and localization. Plant Physiol. 1992;100(1):352–9.
- 35.
Lenassi M, Zajc J, Gostinčar C, Gorjan A, Gunde-Cimerman N, Plemenitaš A. Adaptation of the glycerol-3-phosphate dehydrogenase Gpd1 to high salinities in the extremely halotolerantHortaea werneckiiand halophilicWallemia ichthyophaga. Fungal Biol. 2011;115(10):959–70.
- 36.
Casais-Molina ML, Peraza-Echeverria S, Echevarría-Machado I, Herrera-Valencia VA. Expression ofChlamydomonas reinhardtii CrGPDH2andCrGPDH3cDNAs in yeast reveals that they encode functional glycerol-3-phosphate dehydrogenases involved in glycerol production and osmotic stress tolerance. J Appl Phycol. 2015;28(1):219–26.
- 37.
Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD. Stress tolerance in transgenic tobacco seedlings that overexpress glutathione s-transferase/glutathione peroxidase. Plant Cell Physiol. 2000;41(11):1229.
- 38.
Noctor G, Foyer CH. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016;171(3):1581–92.
- 39.
Schieber M, Chandel Navdeep S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–62.
- 40.
Yoo SD, Cho YH, Sheen J.Arabidopsismesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2(7):1565–72.
- 41.
Jefferson RA. The GUS reporter gene system. Nature. 1989;342(6251):837–8.
- 42.
Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Plant Physiol. 1994;144(3):307–13.
- 43.
Schippers JHM, Nunes-Nesi A, Apetrei R, Hille J, Fernie AR, Dijkwel PP. TheArabidopsisonset of leaf death5 mutation of quinolinate synthase affects nicotinamide adenine dinucleotide biosynthesis and causes early ageing. Plant Cell. 2008;20(10):2909–25.
- 44.
Wei Y, Shen W, Dauk M, Wang F, Selvaraj G, Zou J. Targeted gene disruption of glycerol-3-phosphate dehydrogenase inColletotrichum gloeosporioidesreveals evidence that glycerol is a significant transferred nutrient from host plant to fungal pathogen. J Biol Chem. 2004;279(1):429–35.
- 45.
Queval G, Noctor G. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: application to redox profiling duringArabidopsisrosette development. Anal Biochem. 2007;363(1):58–69.
- 46.
Fryer MJ, Andrews JR, Oxborough K, Blowers DA, Baker NR. Relationship between CO2assimilation, photosynthetic electron transport, and active O2metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol. 1998;116(2):571.
- 47.
Nagalakshmi N, Prasad MNV. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress inScenedesmus bijugatus. Plant Sci. 2001;160(2):291–9.
- 48.
Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photo-oxidative stress responses in leaves. J Exp Bot. 2002;53(372):1249–54.
- 49.
Pei Z-M, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731.
- 50.
Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66.
- 51.
Able AJ, Guest DI, Sutherland MW. Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores ofPhytophthora parasiticavarnicotianae. Plant Physiol. 1998;117(2):491–9.
- 52.
Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive- substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207(4):604–11.
- 53.
Lutts S, Kinet JM, Bouharmont J. NaCl-induced senescence in leaves of rice (Oryza sativaL.) cultivars differing in salinity resistance. Ann Bot-london. 1996;78(3):389–98.
- 54.
Giannopolitis CN, Ries SK. Superoxide dismutase. I. Occurrence in higher plants. J Plant Physiol. 1977;59(2):309–14.
- 55.
Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol.1981;22(5):867–80.
- 56.
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization inArabidopsis. Plant Physiol. 2005;139(1):5–17.
Acknowledgements
We would like to thank Dr. Pradeep Kachroo (University of Kentucky, USA) for providing usAtGPDHc2-KOmutant seeds.
Funding
这是国家重点支持的研究工作nd Development Program of China (2016YFD0101002), Natural Science Foundation of Heilongjiang Province (QC2016036), National Natural Science Foundation of China (31701328), Heilongjiang Bayi Agricultural University Scientific Start-up Found for the Returned Overseas Chinese Scholar (2031011047), Heilongjiang Bayi Agricultural University Key Cultivateing Program (XA2014–01) and Heilongjiang Bayi Agricultural University Graduate Student Innovation Fund Projects (YJSCX2017-Z01). The funding bodies did not play a role in the design of the study and collection, analysis, or interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Author information
Affiliations
Contributions
YZ and ML designed and conceived the experiments. YZ, ML, FW, XL, BY and JW performed the experiments, planted materials and collected samples. LH and CZ analyzed the data and interpreted the results. YZ prepared the manuscript. JX and ZL conceived the experiments and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Authors’ information
Ying Zhao, Bowei Yan and Jinpeng Wei, Ph.D. candidates in crop cultivation and geoponics, Heilongjiang Bayi Agricultural University, Daqing City, Heilongjiang Province, China. Meng Liu and Feng Wang, M.S. candidates in crop cultivation and geoponics, Heilongjiang Bayi Agricultural University, Daqing City, Heilongjiang Province, China. Xin Li, research assistant in Heilongjiang Academy of Agricultural Sciences, Harbin City, Heilongjiang Province, China. Changjiang Zhao and Lin He, assistant professors in College of Agriculture, Heilongjiang Bayi Agricultural University, Daqing City, Heilongjiang Province, China. Zuotong Li and Jingyu Xu, professors in College of Agriculture, Heilongjiang Bayi Agricultural University, Daqing City, Heilongjiang Province, China.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1.Alignment analysis of theZmGPDH1andAtGPDHc2protein sequence (TIF 911 kb)
Additional file 2:
Figure S2.Subcellular localization of pBI121-ZmGPDH1::GFP fusion proteins in rice mesophyll protoplasts.aConfocal micrographs showing localization of GFP and ZmGPDH1-GFP.bConfocal micrographs showing localization of ZmGPDH1-GFP in mesophyll protoplasts expressing a far-red fluorescent protein mkate (TIF 2244 kb)
Additional file 3:
Figure S3.Kinetic analysis of the GPDH activity of ZmGPDH1. (TIF 43 kb)
Additional file 4:
Figure S4.Molecular characterization of theatgpdhc2mutant. a Genomic organization of theatgpdhc2location. b Identification of homozygous mutants. M: DL2000 marker; LP and RP: Forward and reverse primers of target genes; LB: The T-DNA left border primer.cReverse transcription PCR (RT-PCR) ofAtGPDHc2transcripts inatgpdhc2mutants and wild-type (WT)Arabidopsis. (TIF 1762 kb)
Additional file 5:
Figure S5.Phenotype ofZmGPDH1OE lines in response to ABA. a The seeds of WT and OE lines were germinated on half-strength MS plates with or without ABA. b Germination rate of WT and OE lines under different concentrations of ABA treatment at day 5 after imbibitions. c 7-day-old WT and OE seedlings were grown on half-strength MS plates without or with ABA for 7 days. d The fresh weigh and primary root length of WT and OE seedlings after ABA treatment. Asterisks indicate significant differences from WT plants by Student′s t-test (*P < 0.05; **P < 0.01). (TIF 10675 kb)
Additional file 6:
Table S1.The gene ID and primers used in this study. (PDF 86 kb)
Rights and permissions
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, Y., Liu, M., He, L.et al.A cytosolic NAD+-dependent GPDH from maize (ZmGPDH1) is involved in conferring salt and osmotic stress tolerance.BMC Plant Biol19,16 (2019). https://doi.org/10.1186/s12870-018-1597-6
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/s12870-018-1597-6
Keywords
- Glycerol-3-phosphate dehydrogenase
- Glycerol
- Antioxidants
- Redox homeostasis
- Salt stress
- Osmotic stress
- Maize (Zea maysL.)