- Research
- Open Access
- Published:
Flax (Linum usitatissimumL.) response to non-optimal soil acidity and zinc deficiency
BMC Plant Biologyvolume19, Article number:54(2019)
Abstract
Background
Flax (Linum usitatissimumL.) is grown for fiber and seed production. Unfavorable environments, such as nutrient deficiency and non-optimal soil acidity, decrease the quantity and quality of yield. Cultivation of tolerant to stress varieties can significantly reduce the crop losses. Understanding the mechanisms of flax response to the stresses and identification of resistance gene candidates will help in breeding of improved cultivars. In the present work, the response of flax plants to increased pH level and zinc (Zn) deficiency was studied.
Results
We performed high-throughput transcriptome sequencing of two flax cultivars with diverse tolerance to increased pH level and Zn deficiency: Norlin (tolerant) and Mogilevsky (sensitive). Sixteen cDNA libraries were created from flax plants grown under control conditions, increased pH level, Zn deficiency, and both stresses simultaneously, and about 35 million reads were obtained for each experiment type. Unfavorable pH resulted in significantly stronger gene expression alterations compared to Zn deficiency. Ion homeostasis, oxidoreductase activity, cell wall, and response to stress Gene Ontology terms were the most affected by unfavorable pH and Zn deficiency both in tolerant and sensitive flax cultivars. Upregulation of genes encoding metal transporters was identified under increased pH level, Zn deficiency, and both stresses simultaneously. Under Zn deficiency, only in tolerant cultivar Norlin, we revealed the induction of several photosynthesis-related genes and, in this way, this tolerant genotype could overcome unfavorable effects of reduced Zn content.
Conclusions
We identified genes with expression alterations in flax under non-optimal soil acidity and Zn deficiency based on high-throughput sequencing data. These genes are involved in diverse processes, including ion transport, cell wall biogenesis, and photosynthesis, and could play an important role in flax response to the studied stresses. Moreover, genes with distinct expression changes between examined tolerant and sensitive genotypes could determine the mechanisms of flax tolerance to non-optimal soil acidity and Zn deficiency.
Background
Flax (Linum usitatissimumL.) is grown for fiber and seed production and used in textile, pharmaceutical, food, paint, and varnish industries [1].Unfavorable environments, such as nutrient deficiency and non-optimal soil acidity, decrease the quantity and quality of flax yield. Optimal pH level for flax growing is about 5.0–5.5 [2], however, excessive application of lime results in an increase of pH and imbalance of macro- and microelements in soil (especially zinc (Zn) deficiency) that causes physiological depression of flax plants [3].L. usitatissimumgenotypes differ in their tolerance to increased soil pH (7.5 or higher) and Zn deficiency and cultivation of varieties that are tolerant to the stresses can significantly reduce the crop losses [2].Understanding the mechanisms of flax response to increased pH and Zn deficiency and identification of resistance gene candidates will help in breeding of improved cultivars.
High-throughput sequencing is intensively used for studying flax response to diverse stresses, including drought [4], alkalinity and salt [5], excess concentration of aluminum ions [6,7,8], imbalanced nutrition [9,10,11],Fusarium oxysporuminfection [12,13].These studies allowed the revelation of genes with expression alterations under the stresses and identification of processes on which the unfavorable conditions have the greatest impact. Alterations in gene expression under strong alkaline stress (pH 11.6) were investigated in flax plants and genes related to response to biotic stimulus were found to be particularly affected by alkalinity, while photosynthesis-related genes were affected by combined alkaline-salt stress [14].However, transcriptome studies of flax plants under the close to natural conditions of increased pH level (7.5) were not performed and the influence of Zn deficiency on gene expression in flax was not investigated, although, for other plants species, such works aimed at identification of differentially expressed genes were carried out [15,16,17,18,19,20].Zn deficiency leads to disruption of enzyme activity, inhibition of photosynthesis, production of reactive oxygen species (ROS), and increased iron accumulation [21,22].Zn chelators, including nicotianamine, phytosiderophore, glutathione, phytochelatin [23,24,25,26], and transporters, including ZRT (Zinc-Regulated Transporter) - IRT (Iron-Regulated Transporter) - like proteins (ZIPs), Natural Resistance-Associated Macrophage Proteins (NRAMPs), Yellow Stripe-Like (YSL) proteins, Cation Diffusion Facilitator (CDF), heavy metal tolerance (HMA) proteins [27,28,29,30,31,32,33,34),参与植物的锌体内平衡。Alkaline stress disrupts uptake of metal micronutrients, including Zn and Fe, induced production of ROS, and results in alterations in levels of antioxidants, transcriptional factors, phytosiderophores, nicotianamine, photosynthesis-related and heat shock proteins in plants [35,36,37,38,39,40,41,42,43,44].
在目前的研究中,我们进行高通量transcriptome sequencing of flax plants grown under increased pH level, Zn deficiency, and both stresses simultaneously and evaluated gene expression alterations to determine the negative effects of these factors. Since the comparison of responses to the stress for tolerant and sensitive genotypes of the same species is especially important for identification the tolerance mechanisms, we used two flax genotypes with diverse tolerance.
Methods
Plant material
L. usitatissimumcultivars, Norlin and Mogilevsky, which are respectively tolerant and sensitive to unfavorable for flax increased pH level of soil [7], were chosen for the present study. Flax seeds were sterilized with 1% sodium hypochlorite solution for 10 min and then germinated on filter paper in Petri dishes under sterile conditions. Two days later, the germinated seeds were transferred to 15 ml tubes with Murashige-Skoog medium or its modifications with different pH and zinc content: 1) control: pH = 5.5, standard zinc content – 8.6 mg/l ZnSO4·7H2O (pH = 5.5, Zn+); 2) pH = 7.5, standard zinc content (pH = 7.5, Zn+); 3) pH = 5.5, 1000-fold reduced Zn content (pH = 5.5, Zn-); 4) pH = 7.5, 1000-fold reduced Zn content (pH = 7.5, Zn-). About 200 plants of Norlin (tolerant) and Mogilevsky (sensitive) cultivars were grown in a climate chamber for 3 weeks, after which the root tips about 2 cm in length were collected and frozen in liquid nitrogen followed by storage at − 70 °C.
High-throughput sequencing
Total RNA was isolated from roots using RNeasy Plant Mini Kit (Qiagen, USA). In total, 32 RNA samples were isolated from pools of 4–6 plants: two cultivars in four experiment conditions, four biological replicates. The concentration and quality of the isolated RNA were assessed using Qubit 2.0 fluorometer (Life Technologies, USA) and Agilent 2100 Bioanalyzer (Agilent Technologies, USA). For the preparation of cDNA libraries for transcriptome sequencing, two RNA samples from plants of the same cultivar grown under identical conditions were combined in equimolar amounts. As a result, 16 pools of RNA samples were obtained. To prepare the cDNA libraries for high-throughput sequencing, the TruSeq Stranded Total RNA Sample Prep Kit (Illumina, USA) was used. Sixteen cDNA libraries were obtained, the quality of which was evaluated using Agilent 2100 Bioanalyzer (Agilent Technologies). The average length of the cDNA libraries was 270 nucleotides; the adapter dimers were absent; library concentrations were about 13–25 ng/μl. Obtained libraries were mixed in equimolar concentrations and sequenced on NextSeq500 sequencer (Illumina) with 80-nucleotide read length.
Data analysis
First, we removed adapters and trimmed reads with trimmomatic (TRAILING:30 SLIDINGWINDOW:4:20 ILLUMINACLIP:
Obtained reads were mapped to the assembled transcripts using bowtie2 [52] and RSEM [53] and read counts per each transcript were determined. The read counts were analyzed using the edgeR software [54].Transcripts with a counts per million (CPM) less than 2 were filtered out. Multidimensional scaling plot of distances between gene expression profiles in plants of two flax cultivars under the studied conditions was created with edgeR [54].To evaluate expression alterations of each transcript for tolerant and sensitive cultivars, log2(fold change) values were calculated. False discovery rate (FDR) values were obtained by Benjamini-Hochbergp-value adjustment. The gene set enrichment analysis (GSEA) was performed using Goseq (http://bioconductor.org/packages/release/bioc/html/goseq.html), in which top lists of upregulated or downregulated genes were used. Visualization of gene expression alterations in selected GO terms was performed by heatmaps created using edgeR [54].
Results
Transcriptome sequencing and annotation
To study the response of flax plants to non-optimal soil acidity and zinc deficiency, cultivars with different tolerance to the stress were selected on the basis of our previous investigations: tolerant Norlin and sensitive Mogilevsky [7].About 200 plants were grown under control conditions, Zn deficiency (1000-fold reduced Zn content), increased pH level (7.5), and both Zn deficiency and pH = 7.5. After 3 weeks, the phenotype assessment of plants was carried out before collecting material for the sequencing. We observed moderate inhibition of plant growth, leaf bronzing, yellow and necrotic spots on leaves under Zn deficiency in both cultivars. Under increased pH level, growth inhibition and necrosis were more pronounced, especially in sensitive cultivar Mogilevsky. Under both increased pH level and Zn deficiency, strong inhibition of plant growth and necrosis were observed in studied cultivars, however, the symptoms were especially pronounced in Mogilevsky.
We collected plant roots for RNA extraction and prepared cDNA libraries for transcriptome sequencing in duplicate for each cultivar under each of the studied conditions. In total, 16 cDNA libraries were sequenced on the Illumina platform and from 28 to 41 million 80-nucleotide reads were obtained for each sample.
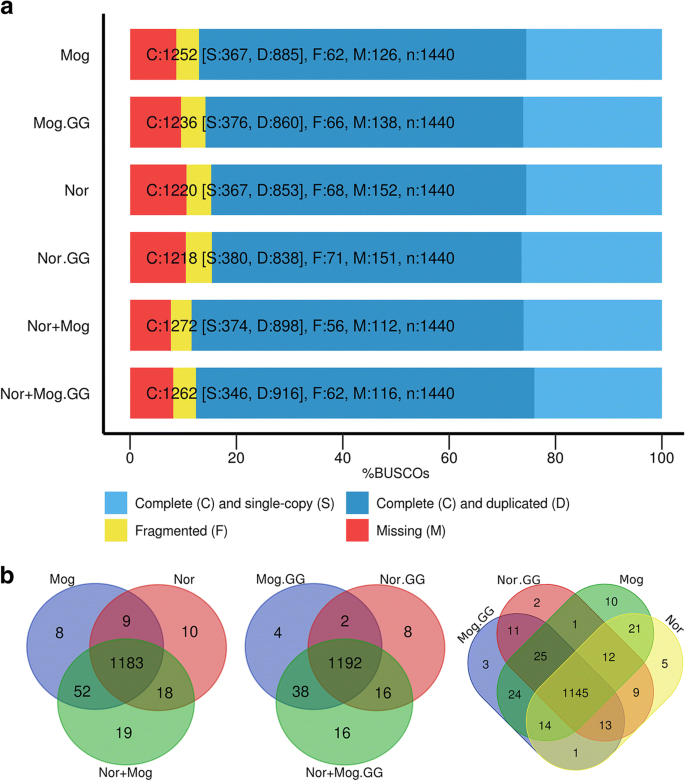
Then, transcriptome assemblies were performed for each cultivar and for the mixed dataset, both genome-guided and non-guided. Genome-guided (GG) assemblies had a bit smaller total length, a greater number of genes but less number of transcripts (Table1). N50 was slightly greater for GG assemblies. Next, we evaluated the completeness of the transcriptome assemblies with BUSCO. Here, GG assemblies did not demonstrate any advantages over non-guided ones. Moreover, non-guided assemblies had a slightly greater number of complete BUSCOs (Fig.1).
Assessment of the completeness of the transcriptome assemblies using BUSCO. Transcripts longer than 500 bp were taken in account. The upper part of figure (a) demonstrates the results of completeness evaluating of the transcriptome assemblies using BUSCO. The bottom part of figure (b) presents three Venn diagrams that illustrate overlaps of the lists of complete BUSCOs between the assemblies. GG – genome-guided assemblies. Mog – cultivar Mogilevsky, Nor – cultivar Norlin, Nor+Mog – mixed dataset of Norlin and Mogilevsky cultivars
The transcriptome assemblies derived for the mixed dataset (Norlin and Mogilevsky cultivars) demonstrated a slight advantage over the individual assemblies in terms of the number of complete BUSCOs (2–4% greater) both for GG and non-guided assemblies. Wherein, the number of genes/transcripts and the total transcriptome length differed dramatically (15–25%). This indicates the presence of orthologous alleles (coming from each cultivar) in the mixed assembly for about 15–25% genes/transcripts. Therefore, we did not use mixed assemblies in the further gene expression analysis between two cultivars because reads from orthologous alleles will be mapped to their own targets in the assembly that will lead to the false-positive differential expression. Hence, for the further analysis, we used non-guided assembly of tolerant cultivar Norlin. Additionally, we checked the completeness of transcriptome assembly containing transcripts > 50 bp (Trinity’s default value) and showed that the number of complete BUSCOs did not increase compared to the assembly containing transcripts > 500 bp, which was used in the present study.
About 90.0–90.4% of Mogilevsky reads and 90.3–90.9% of Norlin reads were successfully mapped to the Norlin assembly using bowtie2 (Additional file1). As can be seen, despite the genetic divergence between the cultivars, a single transcriptome assembly may be correctly used for gene expression quantification.
Expression alterations
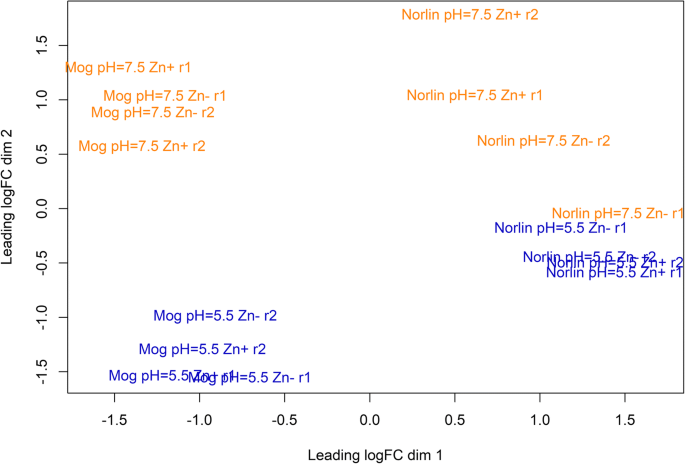
For visualization of the differences in expression profiles between samples, multidimensional scaling (MDS) plot was created (Fig.2). As seen from Fig.2, the samples of Mogilevsky cultivar were separated into two groups: Mogilevsky under pH = 5.5 and Mogilevsky under pH = 7.5. For Norlin cultivar, the separation on the basis of pH level was also observed, but it was not so clear as for Mogilevsky cultivar: one of the biological replicates of “pH = 7.5 Zn-” conditions was close to the subgroup of Norlin under pH = 5.5. We suggest that this discordance of replicates was due to biological variability of plants taken in cDNA library preparation. Nevertheless, it can be concluded that samples of each cultivar under the same pH had close gene expression profiles regardless of Zn content. Thus, unfavorable pH level had a greater effect on flax gene expression compared to Zn deficiency. Cultivar-specific differences in expression profiles were also observed under all studied conditions and samples of the same cultivar were grouped together.
Multidimensional scaling plot of distances between gene expression profiles of two flax cultivars under the studied conditions. The distance between samples indicates their diversity. Cultivars are designated Mog (Mogilevsky) and Norlin, r1 and r2 – biological replicates. Control conditions are indicated pH = 5.5 Zn+; optimal pH level and Zn deficiency – pH = 5.5 Zn-; increased pH level and optimal Zn content – pH = 7.5 Zn+; increased pH level and Zn deficiency – pH = 7.5 Zn-. Blue color – pH = 5.5, orange color – pH = 7.5
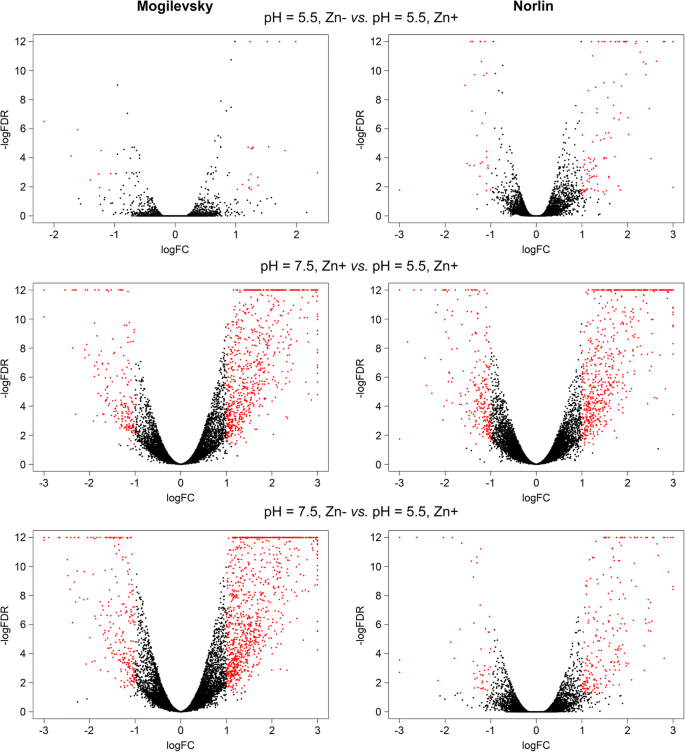
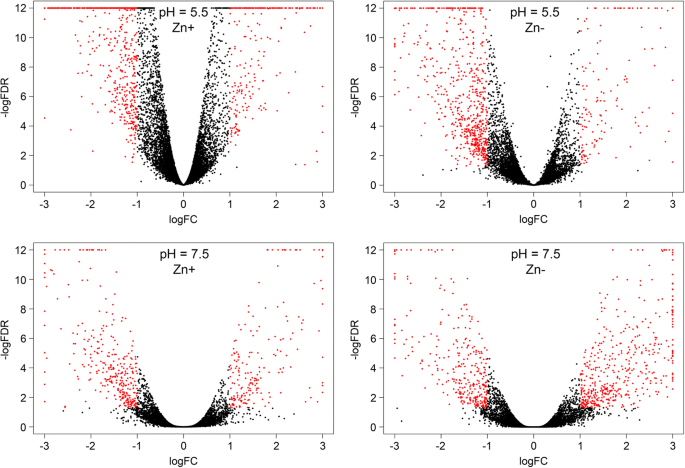
Gene expression analysis was performed and genes with expression alterations were identified for each cultivar under the following stresses: 1) Zn deficiency (pH = 5.5, Zn-), 2) unfavorable pH level (pH = 7.5, Zn+), 3) Zn deficiency and unfavorable pH level (pH = 7.5, Zn-). Genes with significant expression alterations were revealed in each analyzed group (Additional files2,3,4,5,6, and7) and visualization of gene expression changes was performed using Volcano plots (Fig.3) and Venn diagram (Additional file8). Zn deficiency had fewer effects on expression alterations compared to unfavorable pH and expression changes were relatively similar under unfavorable pH level regardless of Zn content ("pH = 7.5, Zn+" and "pH = 7.5, Zn-" conditions). It should be noted that studied cultivars had diverse expression profiles under the same pH level and Zn content both in control and stress conditions (Fig.4). This indicates a significant difference between Norlin and Mogilevsky.
Expression alterations in two flax cultivars under Zn deficiency, unfavorable pH, and both stresses. Volcano plots illustrate the results of differential expression analysis induced by Zn deficiency (pH = 5.5, Zn-), unfavorable pH (pH = 7.5, Zn+), and both stresses simultaneously (pH = 7.5, Zn-) in flax cultivars Norlin (tolerant) and Mogilevsky (sensitive). Each point represents one gene. LogFC – binary logarithm of expression level fold change; LogFDR – decimal logarithm of the false discovery rate. Genes with expression fold change (increase or decrease) > 2 and FDR < 0.05 are marked with red
在亚麻品种Norlin表达差异d Mogilevsky. Volcano plots illustrate the results of differential expression analysis between flax cultivars Norlin (tolerant) and Mogilevsky (sensitive) under control conditions (pH = 5.5, Zn+), Zn deficiency (pH = 5.5, Zn-), unfavorable pH (pH = 7.5, Zn+), and both stresses simultaneously (pH = 7.5, Zn-). Each point represents one gene. LogFC – binary logarithm of expression level fold change; LogFDR – decimal logarithm of the false discovery rate. Genes with expression fold change (increase or decrease) > 2 and FDR < 0.05 are marked with red
Overrepresented GO terms
For identification of the processes in which up- and down-regulated genes under Zn deficiency, increased pH, or both stresses simultaneously are involved, GO enrichment analysis was performed for individual cultivars.
The following GO terms were overrepresented under Zn deficiency and optimal pH = 5.5: related to oxidoreductase activity – for both cultivars in tops 50/100 upregulated genes; related to photosynthesis – for Norlin in tops 50/100 upregulated genes; related to oxidoreductase activity, photosynthesis, and stress response – for Norlin in tops 300/500 upregulated genes; related to cell wall and antioxidant activity – for Mogilevsky in tops 300/500 upregulated genes; related to cell wall – for Norlin in tops 50/100 and 300/500 downregulated genes; related to oxidoreductase activity – for Mogilevsky in tops 50/100 downregulated genes; related to oxidoreductase activity and photosynthesis – for Mogilevsky in tops of 300/359 downregulated genes (Additional files9and10).
控制锌含量和pH = 7.5, following GO terms were overrepresented: related to iron ion homeostasis and vitamin metabolic process – for both cultivars in tops 50/100 upregulated genes; related to heme binding, vitamin metabolic process, iron ion homeostasis, oxidoreductase activity – for both cultivars in tops 300/500 upregulated genes; related to photosynthesis – for Norlin in tops 300/500 upregulated genes; related to extracellular region and heme binding – for Norlin in tops 50/100 and 300/500 downregulated genes; related to oxidoreductase activity and DNA binding – for Mogilevsky in tops 50/100 and 300/500 downregulated genes (Additional files11and12).
Under Zn deficiency and pH = 7.5, the following GO terms were overrepresented: related to zinc ion transmembrane transporter activity – for both cultivars in tops 50/100 upregulated genes (for Norlin FDR was more than 0.05, so overrepresentation was not statistically significant); related to iron ion homeostasis and extracellular region – for Mogilevsky in tops 50/100 upregulated genes; related to response to stress, heme binding, and oxidoreductase activity – for both cultivars in tops 300/500 upregulated genes; related to sulfate transmembrane transporter activity – for Norlin in tops 50/100 downregulated genes; related to cell wall – for both cultivars in tops 300/500 downregulated genes; related to oxidoreductase activity for Mogilevsky in tops 300/500 downregulated genes (Additional files13and14).
Thus, GO terms related to oxidoreductase activity, iron ion homeostasis, cell wall, response to stress, and photosynthesis were the most affected by Zn deficiency and unfavorable pH in flax plants. In tolerant and sensitive cultivars, many processes that were affected by the studied stresses were the same, however, differences in overrepresented categories were also identified. Stimulation of processes involved in photosynthesis was revealed only in tolerant cultivar Norlin in response to Zn deficiency. Possibly, this mechanism provides the tolerance to the stress. Gene expression profiles for GO terms, which were overrepresented under Zn deficiency and pH = 7.5, were visualized by heatmaps and significant differences between studied cultivars were revealed. Heatmap for GO term “response to stress” (ID 0006950) well illustrates diverse gene expression in Norlin and Mogilevsky cultivars both under control and stress conditions and also indicates that unfavorable pH resulted in more significant alterations compared to Zn deficiency (Additional file15).
Discussion
associa亚麻植物生理萧条ted with non-optimal pH (7.5 or higher) and imbalance of macro- and micronutrients in soil and is often caused by liming [3].Application of Zn fertilizers is one of the ways to decrease symptoms of physiological depression, however, this measure is not always effective [7].In the present work, we studied the effects of Zn deficiency, unfavorable pH, and both stresses simultaneously on flax plants with diverse tolerance to physiological depression using transcriptome sequencing.
Under Zn deficiency, inhibition of growth, leaf bronzing, and necrosis were observed for both cultivars - tolerant Norlin and sensitive Mogilevsky. Under pH = 7.5, the same symptoms were much more pronounced, especially in sensitive cultivar Mogilevsky. Effects of both stresses were similar to the effects of pH = 7.5 only. Visualization of expression profiles using MDS plot (Fig.2), volcano plots (Fig.3), and heatmaps (Additional file15) also showed that pH level contribution was more pronounced compared to Zn content. It is known that Zn deficiency can be associated with immobilization of Zn in low available for plant form caused by high soil pH [55,56,57].In flax, Zn deficiency had less negative effects on plants, while the increase in pH level results in crucial damages of plants that indicates greater sensitivity of flax to non-optimal soil acidity.
Some similar trends in expression alterations were revealed in examined flax cultivars under the studied stresses. Upregulation of genes encoding metal transporters (ZIP1, ZIP10, NRAMP5, YSL3) was identified under Zn deficiency, pH = 7.5, and both stresses simultaneously in Norlin and Mogilevsky (Additional files2,3,4,5,6, and7). It is known that particular nutrients, including Zn, are less available for plants at neutral and alkaline soils compared to acidic ones [58,59,60,61].Induction of metal transporters, including ZIP, YSL, and NRAMP, under essential metal deficiency was revealed in a number of plant species [22,62,63,64].We observed upregulation of metal transporters in flax under Zn deficiency and unfavorable pH for the first time and it can be assumed that in this way flax plants compensate the effects of low availability of Zn. Overexpression of peroxidase-encoding genes was identified under pH = 7.5 in both cultivars. Peroxidases are antioxidant enzymes, which role in plant stress response is well known [65,66,67].Induction of peroxidases in flax plants under unfavorable pH is probably associated with oxidative stress and contributes to detoxification of ROS. Genes with distinct expression changes between examined tolerant and sensitive genotypes were identified. Photosynthesis-related GO terms were overrepresented in top lists of 50/100 upregulated under Zn deficiency genes only in tolerant cultivar Norlin. Zn is an essential element of many proteins, which are involved in numerous biochemical processes [22,55,68,69,70].结果表明,锌的缺乏导致depression of photosynthesis [71,72,73,74,75].In flax cultivar Norlin, we revealed the induction of several photosynthesis-related genes under "Zn-" conditions and, possibly in this way, the tolerant genotype overcomes unfavorable effects of Zn deficiency.
It should be noted that tolerant and sensitive cultivars had diverse expression profiles both in control and stress conditions (Fig.4and Additional file15). Therefore, greater tolerance of Norlin cultivar to physiological depression could be associated not only with induction of some genes in response to stress but with constitutive expression of particular genes, which make it more adaptive to the stress.
Thus, expression analysis of genes, which are expressed in flax plants under control conditions and unfavorable pH/Zn deficiency, allowed us to reveal processes that are most affected by the studied stresses. Moreover, identified differences in gene expression between tolerant and sensitive genotypes contribute to the understanding of flax tolerance mechanisms to edaphic stresses.
Conclusions
我们用div研究亚麻品种的响应erse tolerance to unfavorable pH and Zn deficiency using transcriptome sequencing. Under non-optimal pH compared to Zn deficiency, stronger inhibition of plant growth and greater gene expression alterations were revealed for both cultivars. The identified differentially expressed genes are involved in various processes, including ion transport, cell wall biogenesis, oxidoreductase activity, and photosynthesis, and could play an important role in flax response to the studied stresses. Moreover, genes with distinct expression changes between examined tolerant and sensitive genotypes were revealed. These genes could be involved in the mechanisms of flax tolerance to non-optimal soil acidity and/or Zn deficiency.
Abbreviations
- Blast name:
-
gene name of top BLAST hit(s)
- Blast species:
-
organism to which top BLAST hit(s) belongs
- Blast symbol:
-
gene symbol of top BLAST hit(s)
- FDR:
-
adjusted LRTp-value
- Gene ID:
-
gene identifier (generated by Trinity)
- Gene Ontology:
-
Gene Ontology annotation of the top BLAST hit
- KEGG A.Thaliana:
-
KEGG gene/protein identifier (Arabidopsis thaliana) of the top BLAST hit
- KEGG Orthology:
-
KEGG BRITE gene/protein identifier (pan-organismal) of the top BLAST hit
- logCPM:
-
binary logarithm of read counts per million (CPM), average across all the samples
- logFC:
-
binary logarithm of gene expression level fold change between control and stress conditions
- LR:
-
likelihood ratio from edgeR’s likelihood ratio test (LRT)
- Mogilevsky Zn- pH 7.5 r2:
-
CPM per gene in cultivar Mogilevsky under Zn deficiency and unfavorable pH = 7.5 in r2 replicate
- Norlin Zn + pH 5.5 r1:
-
CPM per gene in cultivar Norlin under control conditions in r1 replicate
- PValue:
-
LRTp-value
- Transcript lengths:
-
comma-separated lengths of all predicted transcripts
References
Jhala AJ, Hall LM. Flax (Linum usitatissimum L.): current uses and future applications. Aust J Basic Appl Sci. 2010;4(9):4304–12.
Rozhmina TA, Pavlova LN, Melnikova NV, Golubeva LM. Rol' genofonda l'na v selekcii na adaptivnost'. Uspekhi Sovremennoj Nauki. 2017;1(10):184–9 (in Russian).
Kishlyan NV, Rozhmina TA. Ocenka genofonda lna kulturnogo (Linum usitatissimum L.) po kislotoustojchivosti. Agric Biol. 2010;1:96–103 (in Russian).
Dash PK, Rai R, Mahato AK, Gaikwad K, Singh NK. Transcriptome landscape at different developmental stages of a drought tolerant cultivar of flax (Linum usitatissimum). Front Chem. 2017;5:82.
Yu Y, Wu G, Yuan H, Cheng L, Zhao D, Huang W, Zhang S, Zhang L, Chen H, Zhang J, et al. Identification and characterization of miRNAs and targets in flax (Linum usitatissimum) under saline, alkaline, and saline-alkaline stresses. BMC Plant Biol. 2016;16(1):124.
Dmitriev AA, Krasnov GS, Rozhmina TA, Kishlyan NV, Zyablitsin AV, Sadritdinova AF, Snezhkina AV, Fedorova MS, Yurkevich OY, Muravenko OV, et al. Glutathione S-transferases and UDP-glycosyltransferases are involved in response to aluminum stress in flax. Front Plant Sci. 2016;7:1920.
Dmitriev AA, Kudryavtseva AV, Bolsheva NL, Zyablitsin AV, Rozhmina TA, Kishlyan NV, Krasnov GS, Speranskaya AS, Krinitsina AA, Sadritdinova AF, et al. miR319, miR390, and miR393 Are Involved in Aluminum Response in Flax (Linum usitatissimumL.). Biomed Res Int. 2017;2017:4975146.
Zyablitsin AV, Dmitriev AA, Krasnov GS, Bolsheva NL, Rozhmina TA, Muravenko OV, Fedorova MS, Snezhkina AV, Kudryavtseva AV, Melnikova NV. CAX3 gene is involved in flax response to high soil acidity and aluminum exposure. Mol Biol. 2018;52(4):514–9.
Melnikova NV, Dmitriev AA, Belenikin MS, Koroban NV, Speranskaya AS, Krinitsina AA, Krasnov GS, Lakunina VA, Snezhkina AV, Sadritdinova AF, et al. Identification, expression analysis, and target prediction of flax Genotroph MicroRNAs under Normal and nutrient stress conditions. Front Plant Sci. 2016;7:399.
Melnikova NV, Dmitriev AA, Belenikin MS, Speranskaya AS, Krinitsina AA, Rachinskaia OA, Lakunina VA, Krasnov GS, Snezhkina AV, Sadritdinova AF, et al. Excess fertilizer responsive miRNAs revealed in Linum usitatissimum L. Biochimie. 2015;109:36–41.
Dmitriev AA, Kudryavtseva AV, Krasnov GS, Koroban NV, Speranskaya AS, Krinitsina AA, Belenikin MS, Snezhkina AV, Sadritdinova AF, Kishlyan NV, et al. Gene expression profiling of flax (Linum usitatissimumL.) under edaphic stress. BMC Plant Biol. 2016;16(Suppl 3):237.
Galindo-Gonzalez L, Deyholos MK. RNA-seq Transcriptome Response of Flax (Linum usitatissimumL.) to the Pathogenic Fungus Fusarium oxysporum f. sp. lini. Front Plant Sci. 2016;7:1766.
Dmitriev AA, Krasnov GS, Rozhmina TA, Novakovskiy RO, Snezhkina AV, Fedorova MS, Yurkevich OY, Muravenko OV, Bolsheva NL, Kudryavtseva AV et al. Differential gene expression in response to Fusarium oxysporum infection in resistant and susceptible genotypes of flax (Linum usitatissimumL.). BMC Plant Biol. 2017;17(Suppl 2):253.
Yu Y, Huang W, Chen H, Wu G, Yuan H, Song X, Kang Q, Zhao D, Jiang W, Liu Y, et al. Identification of differentially expressed genes in flax (Linum usitatissimum L.) under saline-alkaline stress by digital gene expression. Gene. 2014;549(1):113–22.
Bandyopadhyay T, Mehra P, Hairat S, Giri J. Morpho-physiological and transcriptome profiling reveal novel zinc deficiency-responsive genes in rice. Funct Integr Genom. 2017;17(5):565–81.
Tiong J, McDonald G, Genc Y, Shirley N, Langridge P, Huang CY. Increased expression of six ZIP family genes by zinc (Zn) deficiency is associated with enhanced uptake and root-to-shoot translocation of Zn in barley (Hordeum vulgare). New Phytol. 2015;207(4):1097–109.
Singh AP, Pandey BK, Deveshwar P, Narnoliya L, Parida SK, Giri J. JAZ repressors: potential involvement in nutrients deficiency response in Rice and chickpea. Front Plant Sci. 2015;6:975.
Li J, Liu B, Cheng F, Wang X, Aarts MG, Wu J. Expression profiling reveals functionally redundant multiple-copy genes related to zinc, iron and cadmium responses in Brassica rapa. New Phytol. 2014;203(1):182–94.
Huang D, Dai W. Two iron-regulated transporter (IRT) genes showed differential expression in poplar trees under iron or zinc deficiency. J Plant Physiol. 2015;186-187:59–67.
刘张李Y, Y, D, X,秦J,通用电气Q,徐L Pan X, Li W, Zhu Y, et al. Spatial-temporal analysis of zinc homeostasis reveals the response mechanisms to acute zinc deficiency in Sorghum bicolor. New Phytol. 2013;200(4):1102–15.
Ricachenevsky FK, Menguer PK, Sperotto RA, Fett JP. Got to hide your Zn away: molecular control of Zn accumulation and biotechnological applications. Plant Sci. 2015;236:1–17.
Sinclair SA, Kramer U. The zinc homeostasis network of land plants. Biochim Biophys Acta. 2012;1823(9):1553–67.
Clemens S, Deinlein U, Ahmadi H, Horeth S, Uraguchi S. Nicotianamine is a major player in plant Zn homeostasis. Biometals. 2013;26(4):623–32.
Suzuki M, Tsukamoto T, Inoue H, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Deoxymugineic acid increases Zn translocation in Zn-deficient rice plants. Plant Mol Biol. 2008;66(6):609–17.
Shanmugam V, Tsednee M, Yeh KC. Zinc tolerance induced by iron 1 reveals the importance of glutathione in the cross-homeostasis between zinc and iron in Arabidopsis thaliana. Plant J. 2012;69(6):1006–17.
Tennstedt P, Peisker D, Bottcher C, Trampczynska A, Clemens S. Phytochelatin synthesis is essential for the detoxification of excess zinc and contributes significantly to the accumulation of zinc. Plant Physiol. 2009;149(2):938–48.
Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci U S A. 1998;95(12):7220–4.
Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci U S A. 2000;97(9):4991–6.
Talke IN, Hanikenne M, Kramer U. Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol. 2006;142(1):148–67.
Kramer U, Talke IN, Hanikenne M. Transition metal transport. FEBS Lett. 2007;581(12):2263–72.
Oomen RJ, Wu J, Lelievre F, Blanchet S, Richaud P, Barbier-Brygoo H, Aarts MG, Thomine S. Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol. 2009;181(3):637–50.
Schaaf G, Schikora A, Haberle J, Vert G, Ludewig U, Briat JF, Curie C, von Wiren N. A putative function for the arabidopsis Fe-Phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant Cell Physiol. 2005;46(5):762–74.
Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M. Phylogenetic and functional analysis of the cation diffusion facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics. 2007;8:107.
Kim YY, Choi H, Segami S, Cho HT, Martinoia E, Maeshima M, Lee Y. AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. Plant J. 2009;58(5):737–53.
Zhang H, Liu XL, Zhang RX, Yuan HY, Wang MM, Yang HY, Ma HY, Liu D, Jiang CJ, Liang ZW: Root Damage under Alkaline Stress Is Associated with Reactive Oxygen Species Accumulation in Rice (Oryza sativaL.). Front Plant Sci 2017, 8:1580.
Guo M, Li S, Tian S, Wang B, Zhao X. Transcriptome analysis of genes involved in defense against alkaline stress in roots of wild jujube (Ziziphus acidojujuba). PLoS One. 2017;12(10):e0185732.
Hsieh EJ, Waters BM. Alkaline stress and iron deficiency regulate iron uptake and riboflavin synthesis gene expression differently in root and leaf tissue: implications for iron deficiency chlorosis. J Exp Bot. 2016;67(19):5671–85.
Meng C, Quan TY, Li ZY, Cui KL, Yan L, Liang Y, Dai JL, Xia GM, Liu SW. Transcriptome profiling reveals the genetic basis of alkalinity tolerance in wheat. BMC Genomics. 2017;18(1):24.
Liu J, Wang Y, Li Q. Analysis of differentially expressed genes and adaptive mechanisms ofPrunus trilobaLindl. under alkaline stress. Hereditas. 2017;154:10.
Takahashi M, Nakanishi H, Kawasaki S, Nishizawa NK, Mori S. Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotechnol. 2001;19(5):466–9.
DuanMu H, Wang Y, Bai X, Cheng S, Deyholos MK, Wong GK, Li D, Zhu D, Li R, Yu Y, et al. Wild soybean roots depend on specific transcription factors and oxidation reduction related genesin response to alkaline stress. Funct Integr Genomics. 2015;15(6):651–60.
王魏张X, L, Z,王t .生理和molecular features of Puccinellia tenuiflora tolerating salt and alkaline-salt stress. J Integr Plant Biol. 2013;55(3):262–76.
Guo M, Wang R, Wang J, Hua K, Wang Y, Liu X, Yao S. ALT1, a Snf2 family chromatin remodeling ATPase, negatively regulates alkaline tolerance through enhanced defense against oxidative stress in rice. PLoS One. 2014;9(12):e112515.
Gong B, Li X, Bloszies S, Wen D, Sun S, Wei M, Li Y, Yang F, Shi Q, Wang X. Sodic alkaline stress mitigation by interaction of nitric oxide and polyamines involves antioxidants and physiological strategies in Solanum lycopersicum. Free Radic Biol Med. 2014;71:36–48.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15(3):R46.
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52.
Simao FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–2.
Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, et al. De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat Protoc. 2013;8(8):1494–512.
Finn RD, Clements J, Arndt W, Miller BL, Wheeler TJ, Schreiber F, Bateman A, Eddy SR. HMMER web server: 2015 update. Nucleic Acids Res. 2015;43(W1):W30–8.
Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40(Database issue):D290–301.
Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9(4):357–9.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC bioinformatics. 2011;12:323.
Robinson MD, McCarthy DJ. Smyth GK: edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40.
Alloway BJ. Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health. 2009;31(5):537–48.
Sharma A, Patni B, Shankhdhar D, Shankhdhar SC. Zinc - an indispensable micronutrient. Physiol Mol Biol Plants. 2013;19(1):11–20.
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173(4):677–702.
Soti PG, Jayachandran K, Koptur S, Volin JC. Effect of soil pH on growth, nutrient uptake, and mycorrhizal colonization in exotic invasive Lygodium microphyllum. Plant Ecol. 2015;216(7):989–98.
Fageria NK, Zimmermann FJP. Influence of pH on growth and nutrient uptake by crop species in an Oxisol. Commun Soil Sci Plant Anal. 1988;29(17–18):2675–82.
Murtaza G, Haynes RJ, Kim KR, Zia MH, Naidu R, Belyaeva ON. Effect of aging biosolids with soils of contrasting pH on subsequent concentrations of cu and Zn in pore water and on their plant uptake. Environ Sci Pollut Res Int. 2012;19(3):636–45.
Ginocchio R, De la Fuente LM, Sanchez P, Bustamante E, Silva Y, Urrestarazu P, Rodriguez PH. Soil acidification as a confounding factor on metal phytotoxicity in soils spiked with copper-rich mine wastes. Environ Toxicol Chem. 2009;28(10):2069–81.
Papierniak A, Kozak K, Kendziorek M, Barabasz A, Palusinska M, Tiuryn J, Paterczyk B, Williams LE, Antosiewicz DM. Contribution of NtZIP1-like to the regulation of Zn homeostasis. Front Plant Sci. 2018;9:185.
Thomine S, Lelievre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H. AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J. 2003;34(5):685–95.
van de Mortel JE, Almar Villanueva L, Schat H, Kwekkeboom J, Coughlan S, Moerland PD, Ver Loren van Themaat E, Koornneef M, Aarts MG. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2006;142(3):1127–47.
Bela K, Horvath E, Galle A, Szabados L, Tari I, Csiszar J. Plant glutathione peroxidases: emerging role of the antioxidant enzymes in plant development and stress responses. J Plant Physiol. 2015;176:192–201.
Pandey S, Fartyal D, Agarwal A, Shukla T, James D, Kaul T, Negi YK, Arora S, Reddy MK. Abiotic stress tolerance in plants: myriad roles of ascorbate peroxidase. Front Plant Sci. 2017;8:581.
Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M: Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection.Genetics and molecular biology2012, 35(4 (suppl)):1011–1019.
Assuncao AG, Persson DP, Husted S, Schjorring JK, Alexander RD, Aarts MG. Model of how plants sense zinc deficiency. Metallomics. 2013;5(9):1110–6.
Rouached H. Recent developments in plant zinc homeostasis and the path toward improved biofortification and phytoremediation programs. Plant Signal Behav. 2013;8(1):e22681.
Assuncao AG, Schat H, Aarts MG. Regulation of the adaptation to zinc deficiency in plants. Plant Signal Behav. 2010;5(12):1553–5.
Yruela I. Transition metals in plant photosynthesis. Metallomics. 2013;5(9):1090–109.
Mattiello EM, Ruiz HA, Neves JC, Ventrella MC, Araujo WL. Zinc deficiency affects physiological and anatomical characteristics in maize leaves. J Plant Physiol. 2015;183:138–43.
Randall PJ, Bouma D. Zinc deficiency, carbonic anhydrase, and photosynthesis in leaves of spinach. Plant Physiol. 1973;52(3):229–32.
Ohki K. Effect of zinc nutrition on photosynthesis and carbonic anhydrase activity in cotton. Physiol Plant. 1976;38(4):300–4.
Brown PH, Cakmak I, Zhang Q. Form and Function of Zinc Plants. In: Robson AD, editor. Zinc in Soils and Plants: Proceedings of the International Symposium on ‘Zinc in Soils and Plants’ held at The University of Western Australia, 27–28 September, 1993. Dordrecht: Springer Netherlands; 1993. p. 93–106.
Acknowledgments
The authors thank All-Russian Research Institute for Flax for the selection and provision of flax seeds. This work was performed using the equipment of “Genome” center of Engelhardt Institute of Molecular Biology (http://www.eimb.ru/rus/ckp/ccu_genome_c.php).
Funding
This work was financially supported by the Russian Science Foundation, grant 16–16-00114. Publication costs were funded by the Russian Science Foundation, grant 16–16-00114.
Availability of data and materials
The datasets generated during the current study are available in the Sequence Read Archive (PRJNA497472).
About this supplement
This article has been published as part ofBMC Plant Biology Volume 19 Supplement 1, 2018: Selected articles from BGRS\SB-2018: plant biology.The full contents of the supplement are available online at//www.cinefiend.com/articles/supplements/volume-19-supplement-1.
Author information
Affiliations
Contributions
AD, TR, and NM conceived and designed the work; TR, AZ, AS, MF, EP, PK, RN, LP, MS, NB, and NM performed the experiments; AD, GK, OM, NB, AK, and NM analyzed the data; AD, GK, and NM wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
伦理批准和同意participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Statistics of mapping of Mogilevsky and Norlin reads to Norlin assembly. (XLSX 10 kb)
Additional file 2:
Gene expression alterations in flax cultivar Mogilevsky under Zn deficiency. (XLSX 6441 kb)
Additional file 3:
Gene expression alterations in flax cultivar Norlin under Zn deficiency. (XLSX 6564 kb)
Additional file 4:
Gene expression alterations in flax cultivar Mogilevsky under pH 7.5. (XLSX 6673 kb)
Additional file 5:
Gene expression alterations in flax cultivar Norlin under pH 7.5. (XLSX 6700 kb)
Additional file 6:
Gene expression alterations in flax cultivar Mogilevsky under Zn deficiency and pH 7.5. (XLSX 6727 kb)
Additional file 7:
Gene expression alterations in flax cultivar Norlin under Zn deficiency and pH 7.5. (XLSX 6580 kb)
Additional file 8:
Venn diagram illustrating the overlaps of the lists of differentially expressed genes (FDR < 0.05, average CPM > 4) induced by Zn deficiency, unfavorable pH, and both stresses simultaneously in flax cultivars Norlin (tolerant) and Mogilevsky (sensitive). (PNG 1264 kb)
Additional file 9:
浓缩去分析的,down-regulated genes in flax cultivar Mogilevsky under Zn deficiency. (XLSX 10861 kb)
Additional file 10:
浓缩去分析的,down-regulated genes in flax cultivar Norlin under Zn deficiency. (XLSX 12506 kb)
Additional file 11:
浓缩去分析的,down-regulated genes in flax cultivar Mogilevsky under pH 7.5. (XLSX 15825 kb)
Additional file 12:
浓缩去分析的,down-regulated genes in flax cultivar Norlin under pH 7.5. (XLSX 16592 kb)
Additional file 13:
浓缩去分析的,down-regulated genes in flax cultivar Mogilevsky under Zn deficiency and pH 7.5. (XLSX 15771 kb)
Additional file 14:
浓缩去分析的,down-regulated genes in flax cultivar Norlin under Zn deficiency and pH 7.5. (XLSX 12492 kb)
Additional file 15:
Patterns of expression of genes participating in response to stress (GO ID 0006950). This heatmap represents Z-scores of normalized read counts per million (CPM) for each gene: from blue (low expression levels) to orange (high expression levels) in flax cultivars Norlin and Mogilevsky. Control conditions are indicated Zn + pH = 5.5; Zn deficiency and optimal pH – Zn- pH = 5.5; optimal Zn content and high pH level – Zn + pH = 7.5; Zn deficiency and high pH level – Zn- pH = 7.5. r1 and r2 – biological replicates. (PNG 864 kb)
Rights and permissions
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Dmitriev, A.A., Krasnov, G.S., Rozhmina, T.A.et al.Flax (Linum usitatissimumL.) response to non-optimal soil acidity and zinc deficiency.BMC Plant Biol19,54 (2019). https://doi.org/10.1186/s12870-019-1641-1
Published:
DOI:https://doi.org/10.1186/s12870-019-1641-1
Keywords
- Flax
- Linum usitatissimum
- Non-optimal acidity
- Zinc deficiency
- Stress response
- High-throughput sequencing