- Research article
- Open Access
- Published:
A repeat region from theBrassica juncea HMA4geneBjHMA4Ris specifically involved in Cd2+binding in the cytosol under low heavy metal concentrations
BMC Plant Biologyvolume19, Article number:89(2019)
Abstract
Background
HMA4 transporters are involved in the transport and binding of divalent heavy metals (Cd, Zn, Pb [lead] and Co [cobalt]). In general, as efflux pumps, HMA4 transporters can increase the heavy metal tolerance of yeast andEscherichia coli.Additional research has shown that the C-terminus of HMA4 contains a heavy metal-binding domain and that heterologous expression of a portion of peptides from this C-terminal domain in yeast provides a high level of Cd tolerance and Cd hyperaccumulation.
Results
We clonedBjHMA4fromBrassica juncea, and quantitative real-time PCR analysis revealed thatBjHMA4was upregulated by Zn and Cd in the roots, stems and leaves. Overexpression ofBjHMA4dramatically affects Zn/Cd distribution in rice and wheat seedlings. Interestingly, BjHMA4 contains a repeat region named BjHMA4R within the C-terminal region; this repeat region is not far from the last transmembrane domain. We further characterized the detailed function of BjHMA4R via yeast andE. coli实验。Notably, BjHMA4R greatly and specifically improved Cd tolerance, and BjHMA4R transformants both grew on solid media that contained 500 μM CdCl2and presented improved Cd accumulation (approximately twice that of wild-type [WT] strains). Additionally, visualization via fluorescence microscopy indicated that BjHMA4R clearly localizes in the cytosol of yeast. Overall, these findings suggest that BjHMA4R specifically improves Cd tolerance and Cd accumulation in yeast by specifically binding Cd2+in the cytosol under low heavy metal concentrations. Moreover, similar results inE. coliexperiments corroborate this postulation.
有限公司nclusion
BjHMA4R can specifically bind Cd2+in the cytosol, thereby substantially and specifically improving Cd tolerance and accumulation under low heavy metal concentrations.
One-sentence summary
BjHMA4R can specifically bind Cd2+in the cytosol, thereby substantially and specifically improving Cd tolerance and accumulation under low heavy metal concentrations.
Background
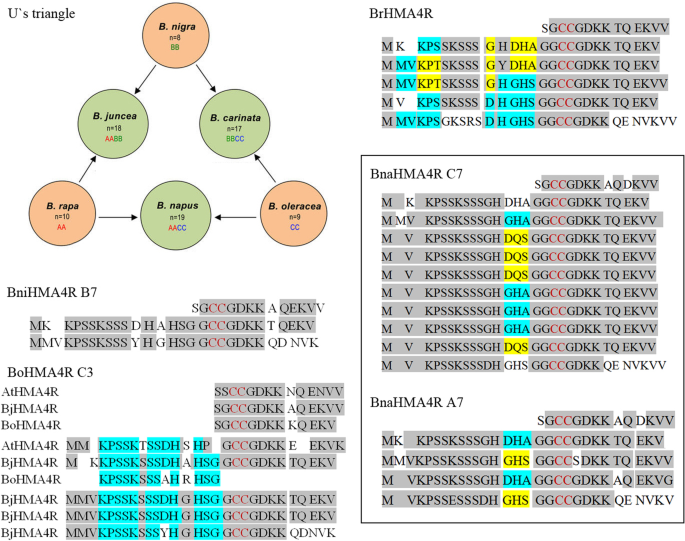
Brassica juncea, commonly known as Indian mustard, is a species of mustard plant. According to U’s triangle,B. juncea(AABB genome, 2n = 4× = 36) is the allotetraploid derived from the diploid speciesB. rapa(AA, 2n = 2× = 20) andB. nigra(BB, 2n = 2× = 16) and arose by natural hybridization and chromosome doubling [1,2]。This mustard plant can be used in phytoremediation to remove heavy metals and metalloids, such as cadmium (Cd), lead (Pb), zinc (Zn), cobalt (Co), nickel (Ni), copper (Cu), chromium (Cr), arsenic (As), cesium (Cs), and uranium (U), from the soil in hazardous waste sites. Thus, Indian mustard (Brassica juncea), a high-biomass-producing plant, has been broadly used as a model system to investigate the physiology and biochemistry of metal and radionuclide accumulation in plants [3,4,5,6]。However, the molecular mechanism of the hyperaccumulation phenotype remains unclear. HMA4 may play a significant role in the hyperaccumulation-related characteristics of hyperaccumulator species, at least within Brassicaceae plants.
HMA4 (heavy metal ATPase 4) is a member of the type 1Bheavy metal-transporting P-type ATPase subgroup, whose members transport the divalent cations Cd, Pb (lead), Zn, and Co (cobalt) [7,8]。To date, this divalent class of transports exists only in prokaryotes and plants. There are four members inArabidopsis, HMA1, HMA2, HMA3, and HMA4. AtHMA2 and AtHMA4 both have long C-terminal hydrophilic extensions that seem specific to plant P1B-ATPases [9,10], and the C-terminal extension of AtHMA4, which includes 44 cysteine (Cys) and a terminal stretch of 11 consecutive histidine residues, is longer than that of AtHMA2. Moreover, the N-terminal regions of AtHMA2 and AtHMA4 are highly similar, and they exhibit similar expression patterns (predominantly in the vascular tissues of roots, stems and leaves) and subcellular localization (the plasma membrane) [11]。
The sequence of TcHMA4, which is an ortholog of AtHMA4 fromThlaspi caerulescens, is 76% similar to that of AtHMA4, has more divergent C-teminal sequence and shares only 43% identity with the counterpart in AtHMA4. Interestingly, two truncated peptides from the TcHMA4 C-terminal domain were expressed in yeast, which resulted in an extremely high level of Cd tolerance and hyperaccumulation [12,13,14]。There are 3HMA4gene copies in theArabidopsis hallerigenome, and the activity of all threeAhHMA4promoters is much higher than theAtHMA4promoter activity, leading to the highHMA4transcript levels inA. halleri.Moreover, QTL analysis, physiology experiments and reverse genetic studies have demonstrated thatAhHMA4has played a major role in the natural selection of Zn hyperaccumulation and associated Cd and Zn hypertolerance inA. halleri[15]。
Taken together, the striking differences in gene coding sequence, especially at the 3′-end,cis-regulatory regions and gene copy numbers observed between the hyperaccumulatorT. caerulescensandA. halleriandA. thaliana, the latter of which is a close nontolerant nonaccumulator relative, suggest thatHMA4was subjected to positive selection during adaptive evolution. These differences led to changes inHMA4expression levels and physiological function predominantly in substrate specificity and C-terminal heavy metal binding capacity. Generally, HMA4s have a conserved N-terminus and more divergent C-terminus. The functional domains of the N-terminus have been well characterized by sequence alignments and experiments, but the positions and functions of the C-terminal domains have not been well studied.
In the present study, we clonedBjHMA4fromB. juncea.有限公司mpared with other HMA4s, BjHMA4 had a more divergent and long C-terminal hydrophilic extension and repeat region (named BjHMA4R, which had 4 CC dipeptides [Cys pairs]). We then characterized BjHMA4R in detail to investigate its function. Our results show that BjHMA4R facilitates a high degree of heavy metal tolerance in yeast via its high binding activity and possibly interacts with other heavy metal transporters as a chaperone, resulting in increased heavy metal flux.
Results
Characterization ofBjHMA4
Isolation ofB. junceaHMA4 cDNA
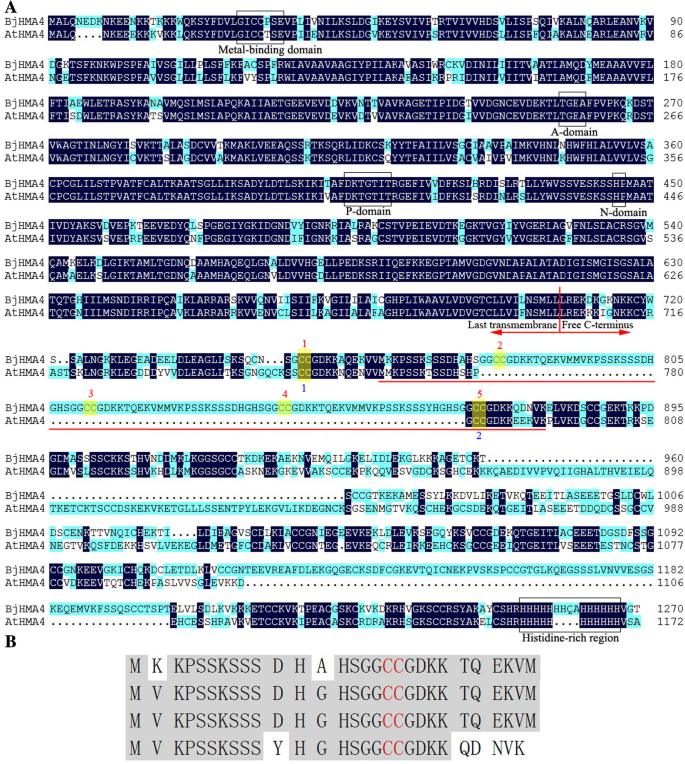
Based on homology cloning strategies and the RACE technique, theBjHMA4gene cloned fromB. junceawas submitted to GenBank (accession No. JQ673430) and was found to encode 1271 amino acids and have six transmembrane domains (predicted using the TMHMM 2.0 server,http://www.cbs.dtu.dk/services/TMHMM/).The deduced amino acid sequence of BjHMA4 is 61 and 64% identical to that of AtHMA4 (fromA. thaliana) and TcHMA4 (fromT. caerulescens), respectively. The low sequence similarity was due to the high divergence of the BjHMA4-C region, i.e., the sequence after the last transmembrane domain. Alignment with AtHMA4 indicated that BjHMA4 contains 5 highly conserved domains of HMA4: the metal-binding domain (binds heavy metals), A-domain (which includes the smaller cytoplasmic loop region; this domain functions as the ‘actuator’ of the gating mechanism that regulates Ca2+binding and release), P-domain (which is homologous to members of the catalytic HAD family of enzymes; this domain contains the phosphorylation site), N-domain (contains the ATP-binding site) and histidine-rich region (which may also participate in heavy metal binding) (Fig.1a) [16]。Interestingly, the BjHMA4 C-terminal region contains an apparent 4-repeat region (named BjHMA4R) that is not far from the last transmembrane domain (Fig.1a), which increases the number of CC dipeptides (Cys pairs) in BjHMA4 (AtHMA4 contains 13, TcHMA4 contains 16, and BjHMA4 contains 17) (Fig.1b).
aSequence alignment of BjHMA4 fromB. junceaand theArabidopsishomolog AtHMA4. The deduced amino acid sequences of BjHMA4 and AtHMA4 (accession No. 064474) shown were aligned using DNAMAN. The dark blue shading indicates identical residues. Several domains common to P1B-type ATPases are indicated by boxes. Additionally, the numerous Cys residue pairs within the C-terminal region are indicated by numbers. The repeat region in BjHMA4 (BjHMA4R) is underlined in red.b重复地区BjHMA4 (BjHMA4R)。半胱氨酸残基pairs are shown in red
Expression profiles ofBjHMA4
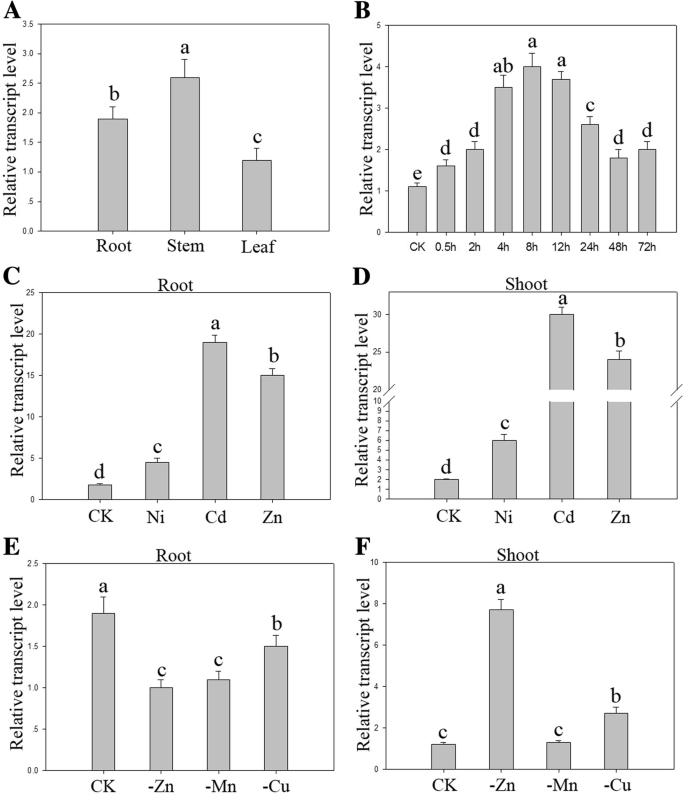
The expression ofBjHMA4was studiedin plantavia quantitative real-time PCR analysis under stringent conditions. The primers used were designed from the 3` region of the cDNA, which was predicted to be the most divergent among the differentHMAs.There was no significant difference inBjHMA4expression among the roots, stems and shoots at the seedling stage; however, the expression in the roots increased upon treatment with 600 μM ZnCl2and subsequently decreased to normal levels depending on the treatment duration. The level of BjHMA4 transcripts was upregulated and peaked after 8 h of treatment; this level was approximately four times greater than normal levels. The BjHMA4 expression in the roots and shoots increased approximately 10–20-fold under Zn and Cd stresses and slightly increased under Ni stress. Under Zn, Mn, Cu deficiency stresses, BjHMA4 expression increased approximately 4-fold under Zn deficiency stress only in the shoots (Fig.2).
Expression profiles ofBjHMA4inB. juncea.aExpression analyses ofBjHMA4genes under normal conditions. The total RNA was extracted from the roots, stems, and leaves of three-week-oldB. junceaplants.bThe expression levels ofBjHMA4in the roots under different ZnCl2treatment periods.c,dExpression analyses ofBjHMA4genes in the roots and shoots in response to heavy metal treatments. Three-week-old WTB. junceaplants were exposed to 400 μM NiCl2, 200 μM CdCl2, or 2000 μM ZnCl2for 6 h.e,fExpression analyses ofBjHMA4genes in the roots and shoots under Mn-, Cu- or Zn-deficient conditions. Two-week-oldB. junceaplants were transferred to Mn-, Cu- or Zn-depleted media for one week. The data represent the means of three biological replicates. Different letters above the columns indicate significant differences (P < 0.05) between seedling groups under the same stress treatment
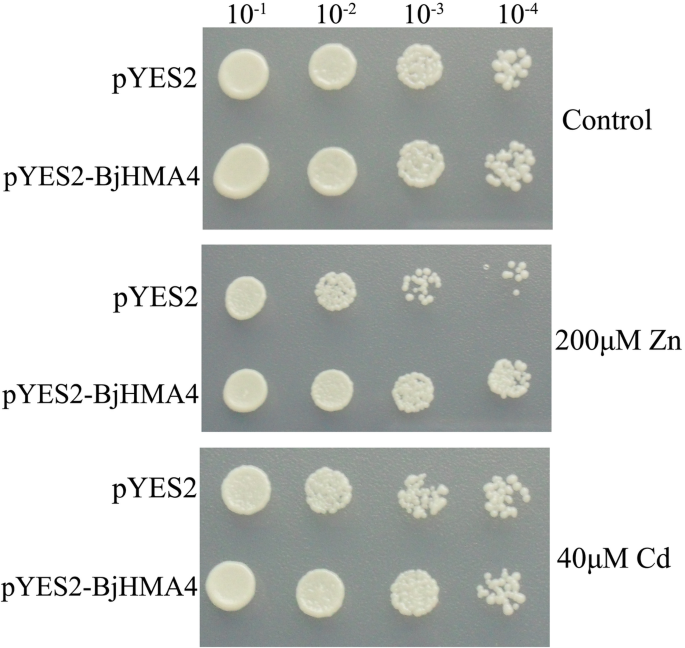
Overexpression ofBjHMA4only slightly increases Zn/cd tolerance in yeast
The pYES2 and pYES2-BjHMA4 vectors were transformed into yeast YK44 (ura3–52 his3–200,△ZRC,△有限公司t1, mating type a), which is sensitive to Zn and Cd, to investigate whether BjHMA4 could transport and bind these metals. We observed that compared with the empty vector, the pYES2-BjHMA4-transformed YK44 grew fairly well on YPD plates containing 200 μM ZnCl2and 40 μM CdCl2(Fig.3).
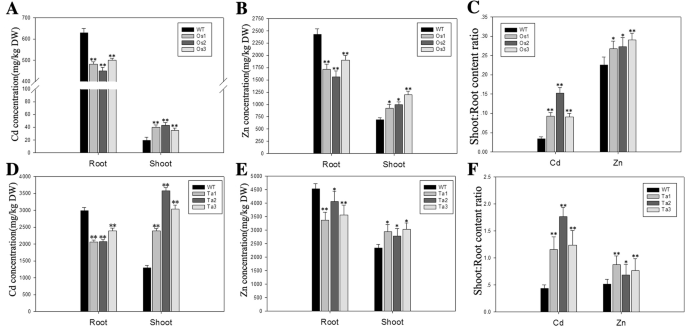
Overexpression ofBjHMA4dramatically affects Zn/cd distribution in rice and wheat seedlings
The results of semi-reverse transcription-PCR and real-time PCR analysis verified BjHMA4 expression in the transgenic rice and wheat (Additional file1: Data S1). The overexpression of BjHMA4 in rice and wheat, which was driven by the ubiquitin and Actin1 promoter respectively, affected the Zn/Cd distribution of the transgenic plants. The Zn and Cd contents decreased significantly in the roots but increased in the shoots of the transgenic rice and wheat. Thus, the root-shoot Zn/Cd translocation significantly increased in BjHMA4 transgenic rice and wheat (Fig.4).这些结果表明,BjHMA4可能范围n as an efflux pump in the vascular tissues.
The Zn/Cd content in theBjHMA4transgenic and corresponding wild-type (WT) plants. Shown are cadmium (a,d) and zinc concentrations (b,e) in the roots and the shoots and the shoot:root ratios (c,f) of cadmium and zinc concentrations in 3-week-old rice and wheat transformed withBjHMA4and WT rice and wheat grown hydroponically for 2 weeks and subsequently exposed to 200 μM CdCl2or 2 mM ZnCl2for a week. The data are expressed as the mean ± SE of three replicates; * and ** indicate significance at 5 and 1% levels (evaluated by Student’sttest), respectively
Characterization ofBjHMA4R
Homologous sequences of BjHMA4R only exist in Brassicaceae
To determine whether other plants possess homologous sequences to BjHMA4R, we used theBjHMA4cDNA sequence as a querying probe to search against other plant genomes in GenBank by the online BLAST tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi).The results showed that highly homologous sequences existed only in Brassicaceae.Carica papaya(genome taxid: 3649), which belongs to Brassicales, has only an N-terminal homologous sequence to BjHMA4, not C-terminal. Similar results were obtained fromMedicago truncatula(taxid: 3880),Solanum lycopersicum(taxid: 4081), andNicotiana tabacum(taxid: 4907). Interestingly, plants that belong toBrassica, especially the 6 species that belong to U’s triangle, had highly homologous sequences. Among the 3 diploids,B. nigra(BB, genome taxid: 3710) had 2 repeats located on chromosome B7 (sequence ID: CM004497.1),B. rapa(AA, taxid: 3711) had 5 repeats located on chromosome A7 (sequence ID: NC_024801.1), andB. oleracea(CC, taxid: 3712) had a partial repeat located on chromosome C3 (sequence ID: NC_027750.1) (Fig.5).Among the 3 allotetraploids, onlyB. napushad homologous sequences in (AACC, genome taxid: 3708): chromosome A7 (sequence ID: NC_027763.2) had 4 repeats, and chromosome C7 (sequence ID:NC_027773.2) had 10 repeats. We speculate thatB. juncea(AABB, taxid: 3707) andB. carinata(BBCC, taxid: 52824) showed no homologous sequences because their genome sequences have not been fully assembled yet (Fig.5).Arabidopsis thalianaandArabidopsis halleriboth had 1 repeat. TcHMA4 had 1 repeat, which had more different amino acid residues than the others. There are 6 strongly conserved amino acid residues (CCGDKK) in the Brassicaceae plants as analyzed here. The CC in that sequence is the first cystine pair in the C-terminus, and this six-residue sequence is part of the repeat and is at the front of the repeats in the Brassicaceae plants that we analyzed (Fig.5and Additional file2: Data S2) .
Subcellular localization of BjHMA4R
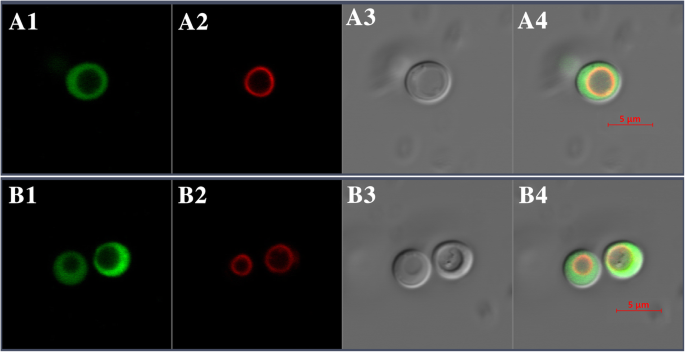
BjHMA4R clearly localizes to the yeast cytosol. When cells in the stationary phase were analyzed, green fluorescence from the expression of pUG36-BjHMA4R was distributed throughout the cytosol. To verify the localization of the fusion protein in the cytosol, the vacuolar membrane was stained for a short period of time with the lipophilic dye FM4–64, which selectively stains the yeast vacuolar membrane. The merged GFP and FM4–64 images demonstrated that GFP-tagged BjHMA4R was localized in the yeast cytosol (Fig.6).Different yeast strains, including BY4741 andend3(data not shown), were used to determine the subcellular localization of BjHMA4R, and the same result was obtained with both strains. This result is consistent with that via the bioinformatics-based hydropathy analysis method.
Subcellular localization of pUG36 (A1-A4) and pUG36-BjHMA4R (B1-B4) fusion proteins in yeast strain BY4741. Yeast cells expressing pUG36 and pUG36-BjHMA4R were cultured in SD-URA media supplemented with Glu; however, when the cells reached the stationary phase, they were then transferred to SD-URA-Met media supplemented with Glu for 12 h. A1 and B1, eGFP fluorescence signals; A2 and B2, vacuoles; A3 and B3, bright-field images; A4 and B4, overlay of GFP fluorescence signals, vacuoles and bright-field images. Bars = 5 μm
Expression ofBjHMA4Rin yeast andE. coliprovides heavy metal tolerance
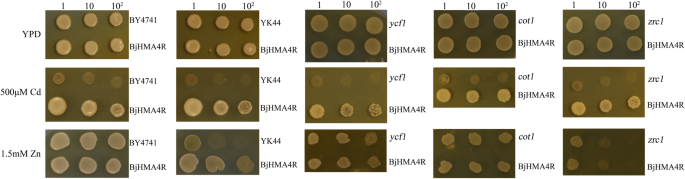
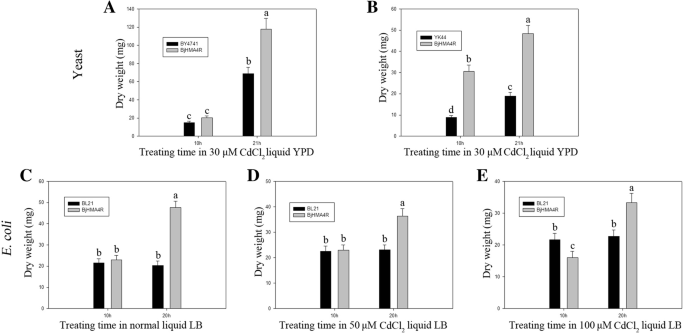
To investigate the substrate specificity of BjHMA4R and its functional properties, the protein was expressed in BY4741 (WT),ycf1,zrc1,cot1andzrc1&cot1(YK44) yeast strains. All the strains were transformed withBjHMA4R,导致一个重要的Cd宽容but not Zn, Ni, Co or Pb tolerance (Additional file3: Data S3). As shown in Fig.7, the growth of the BY4741 and YK44 cells expressing the empty pYES2 vector was strongly inhibited by the inclusion of 500 μM Cd in the solid YPD media, while compared with control cells, yeast cells expressingBjHMA4Rgrew significantly more on substantially high levels of Cd (500 μM) in solid YPD media and grew better in liquid YPD media that contained 30 μM Cd (Fig.8).The dry weight of the BY4741 and YK44 transformants expressing BjHMA4R were both significantly higher than that of the control cells after 21 h of culture, and the dry weight increased approximately 1-fold (Fig.8).These findings indicated that the growth superiority of yeast transformants withBjHMA4Rwas apparent under Cd stress, particularly for the Cd-sensitive YK44 double mutant. In yeast, ZRC1 and COT1 are members of the CDF cation transporter family and are localized in the vacuolar membrane. ZRC1 is mainly responsible for transporting Zn2+and Cd2+, and COT1 is mainly responsible for transporting Co2+from the cytosol into the vacuole [17]。Thus, the YK44 double mutant (cot1&zrc1) is sensitive to Zn, Cd and Co stress. The results of this experiment showed that the substrate specificity of ZRC1 and COT1 was not very high, and thezrc1andcot1strains were not sensitive to Zn under 1.5 mM Zn stress; however, the YK44 strain was sensitive to Zn. Interestingly, BjHMA4R-transformed YK44 could rescue the Zn sensitivity phenotype of YK44 under 1.5 mM Zn stress (Fig.7).
Growth of yeast cells expressingBjHMA4Runder Cd stress. BY4741, YK44,ycf1,cot1andzrc1transformants expressed pYES2 (negative control) andBjHMA4R, respectively. The cultures were adjusted to an OD600of 1 and were serially diluted 10-fold in water. Five-microliter aliquots of each dilution were spotted either on nonselective YPD plates or on YPD plates supplemented with 500 μM CdCl2and 1.5 mM ZnCl2.经过3天的孵化30°C,这些盘子were imaged. The dilutions are indicated in the above figure, and three individual clones of each yeast transformant were analyzed
Dry weight of yeast andE. coliexpressingBjHMA4R.a,bYeast BY4741 and YK44 cells transformed with a pYES2 plasmid and a pYES2 plasmid that harboredBjHMA4Rwere grown in liquid YPD medium supplemented with 30 μM CdCl2.The cells were incubated at 30 °C for 10 h and 21 h. Fifteen milliliters of yeast fluid was collected by centrifugation and then dried at 95 °C for 48 h for determination of the dry weight.c,d,eE. coliBL21 cells transformed with a pEASY-Blunt E1 expression plasmid or with a pEASY-Blunt E1 expression plasmid that harboredBjHMA4Rwere grown in liquid LB medium supplemented with normal LB, 50 μM CdCl2and 100 μM CdCl2.The cells were incubated at 37 °C for 10 h and 20 h. Fifteen milliliters ofE. coli流体是由离心收集然后dried at 95 °C for 48 h to determine the dry weight. The results are the means ± SEs of four independent experiments performed with four different colonies. Different letters above the columns indicate significant differences (P < 0.05) between different cell groups under the same stress treatment
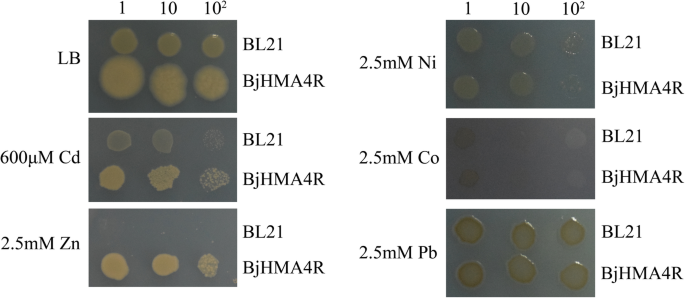
Similar results were obtained from ourE. coli实验。有限公司mpared with control cells, strain BL21 cells transformed with BjHMA4R exhibited significant levels of Cd (600 μM) and Zn (2.5 mM) tolerance and greater growth in solid LB media but not Ni, Co or Pb tolerance (Fig.9).The dry weights of the BL21 transformants expressing BjHMA4R were significantly higher than those of the control cells after 20 h of culture, especially when cultured in normal and 50 μM CdCl2-supplemented liquid LB media (Fig.8).
Growth ofE. coliexpressingBjHMA4R.BL21 transformants contained a pEASY-Blunt E1 expression vector (negative control) andBjHMA4R, respectively. The cultures were adjusted to an OD600of 1 and were serially diluted 10-fold in water. Five-microliter aliquots of each dilution were spotted either on nonselective LB plates or on LB plates supplemented with 600 μM CdCl2and 2.5 mM ZnCl2,Ni(NO3)2, Co(NO3)2and Pb(NO3)2.After 1 day of incubation at 37 °C, the plates were imaged. The dilutions are indicated in the above figure, and three individual clones of eachE. colitransformant were analyzed
Heavy metal accumulation in control cells and BjHMA4R-transformed yeast andE. colicells
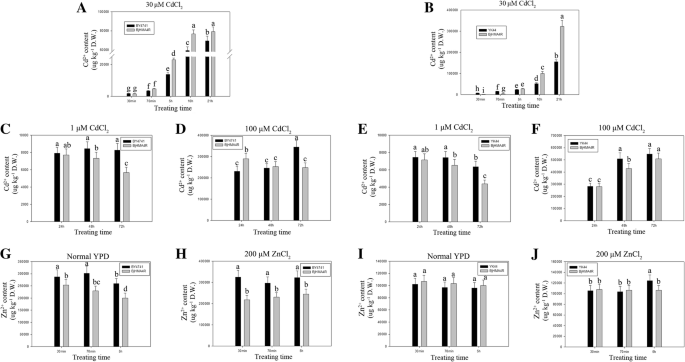
Metal accumulation experiments were conducted with WT yeast (BY4741) and double mutant yeast (YK44) cells expressing empty pYES2 vectors as well as yeast cells expressingBjHMA4R.These experiments involved both yeast strains and genotypes incubated in liquid YPD medium supplemented with either 30 μM CdCl2or 200 μM ZnCl2and harvesting yeast cells after 30 min, 70 min, 5 h, 10 h, and 21 h of metal accumulation. In general, the metal accumulation profiles of the BY4741 and YK44 strains distinctly differed from those of the control and theBjHMA4R-transformed yeast strain at different treatment time points (Fig.10a, b). As shown in Fig.10,BjHMA4R-transformed BY4741 always accumulated more Cd than did BY4741 at every time point, and the maximum difference emerged after 5 h of treatment.BjHMA4R-transformed BY4741 accumulated approximately 70% more Cd than did the control. With respect to YK44,BjHMA4R-transformed YK44 accumulated more than 4-fold less Cd than did the control YK44 at the 30 min time point and 2.7-fold less Cd at 70 min. However,BjHMA4R-transformed YK44 accumulated more Cd than did the control YK44 after 5 h of treatment, and the difference was more clear.BjHMA4R-transformed YK44 accumulated approximately twice as much Cd as did the control YK44 after 21 h of treatment (Fig.10b). There was no apparent difference in Cd content between theBjHMA4R-transformed and control strains under low and high concentrations of Cd (1 and 100 μM CdCl2) and long periods of stress (24 h, 48 h, and 72 h) (Fig.10c, d, e, f). There was no significant difference in Zn accumulation between theBjHMA4R-transformed cells and control cells under 200 μM ZnCl2stress, but the Zn accumulation apparently differed between BY4741 and YK44; BY4741 accumulated approximately 1.5-fold more Zn than did YK44(Fig.10g, h, i, j). Similar results were obtained by 200 μM Ni(NO3)2, Co(NO3)2, and Pb(NO3)2stress treatments. BY4741 accumulated more Co and less Ni than did YK44, but the Pb content was about the same (Additional file4: Data S4a,b,d,e,g,h).
Cd and Zn contents of yeast expressingBjHMA4R.Yeast BY4741 and YK44 cells transformed with a pYES2 plasmid or a pYES2 plasmid that harboredBjHMA4Rwere grown in normal liquid YPD medium overnight. Then, they were supplemented with 30 μM CdCl2(a,b), 1 μM CdCl2(c,e), 100 μM CdCl2(d,f), normal medium (g,i), or 200 μM ZnCl2(h,j).The cells were incubated at 30 °C for 30 min, 70 min, 5 h, 10 h or 21 h. The metal contents of the samples were analyzed with inductively coupled plasma-mass spectrometry (ICP-MS). The results are the means ± SEs of four independent experiments performed with four different colonies. Different letters above the columns indicate significant differences (P < 0.05) between cell groups under the same stress treatment
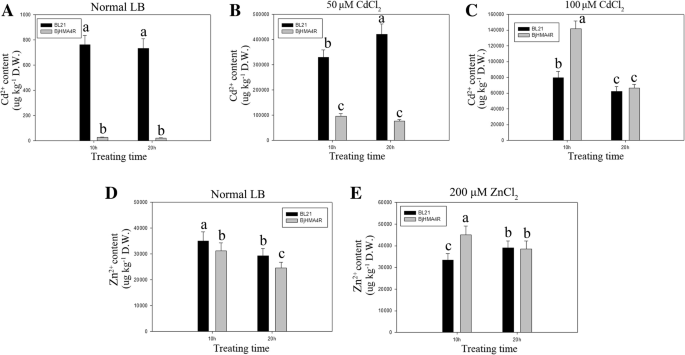
With respect toE. coli,BjHMA4R-transformed BL21 accumulated less Cd than did the control BL21 under normal culture conditions and under low-concentration Cd (50 μM CdCl2) stress(Fig.11a, b). There was no apparent difference in Cd content between theBjHMA4R-transformed and control strains under high concentrations of Cd (100 μM CdCl2) and long periods of stress (21 h) (Fig.11c). Interestingly, both BL21 genotypes (BjHMA4R-transformed and control ones) presented approximately 1-fold more Cd accumulation under 50 μM CdCl2stress than under 100 μM CdCl2stress (Fig.11b, c). Zn accumulation ofBjHMA4R-transformed BL21was slightly but significantly less than did the control BL21 under normal culture conditions(Fig.11d), however,BjHMA4R-transformed BL21 had significantly more Zn than did the control under 200 μM ZnCl2and relatively short periods of stress (10 h) (Fig.11e). There were no significant differences in Ni, Co, or Pb accumulation betweenBjHMA4R-transformed and control BL21 cells under 200 μM Ni(NO3)2, Co(NO3)2, or Pb(NO3)2stress (Additional file4: Data S4c,f,i).
Cd and Zn contents ofE. coliexpressingBjHMA4R.E. coliBL21 cells transformed with a pEASY-Blunt E1 expression plasmid or a pEASY-Blunt E1 expression plasmid that harboredBjHMA4Rwere grown in normal liquid LB medium overnight, then supplemented with 50 μM CdCl2(b), 100 μM CdCl2(c), 200 μM ZnCl2(e) and normal LB (a,d).The cells were incubated at 37 °C for 10 h and 20 h. The metal contents of the samples were analyzed with inductively coupled plasma-mass spectrometry (ICP-MS). The results are the means ± SEs of four independent experiments performed with four different colonies. Different letters above the columns indicate significant differences (P < 0.05) between cell groups under the same stress treatment.
Cd2+flux from transformed yeast andE. colicells in response to CdCl2stress treatments
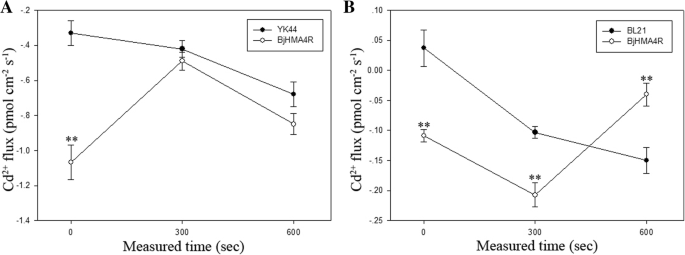
Using the NMT (Non-invasive Micro-test Technology) technique, we measured Cd2+flux across cell membranes. In response to 30 μM CdCl2stress, BjHMA4R changed the signatures of Cd2+flux of transformed cells in comparison with control cells.
Overexpression ofBjHMA4Rcan increase Cd2+influx of transgenic yeast andE. colicells when just under Cd stress compared with control cells (Fig.12).With the extension of treatment time, the Cd2+flux of yeast cells tended to be consistent (Fig.12a), and the transgenic cells ofE. colihad significantly Cd2+efflux compared with control cells (Fig.12b). The above results are basically consistent with those of metal content analysis.
Detection of BjHMA4R activity by Cd2+flux measurements. Transgenic BjHMA4R and control cells were exposed to 30 μM Cd2+and measurements were taken for 600 s, using a vibrating probe, after the flux became ready.ayeast cells, bE. colicells.The data are expressed as the mean ± SE of three replicates; * and ** indicate significant levels at 5 and 1% (evaluated by Student’sttest), respectively
Discussion
HMA4 C-terminus may have important physiological functions
In their N-terminal regions, AtHMA4 and TcHMA4 present the degenerate MBD domain sequence G25ICCTSE31[12]。This domain is very conservative, and all substitutions affecting this degenerate core sequence had deleterious effects on the AtHMA4 complementation efficiency [11]。However, the N-terminal MBD domain of BjHMA4 bears the sequence G29ICCPSE35(Fig.1); substitutions of T with P should not affect the activity of BjHMA4 because T and P have similar structures and chemical properties, and the R-groups of both are uncharged. The A-domain, P-domain and N-domain sequences are identical to those of AtHMA4 (Fig.1).However, BjHMA4 bears a longer histidine-rich region than does AtHMA4 and TcHMA4; AtHMA4 and TcHMA4 present 11 and 9 histidine stretches within their last C-terminal portion, respectively. BjHMA4 bears 14 histidines, and two contiguous Q and A residues were inserted into the middle of the histidine-rich region (Fig.1).The highly divergent sequence of BjHMA4, especially within the C-terminal region, suggests that HMA4 was subjected to positive selection during adaptive evolution and may play a significant role in the hyperaccumulation-related characteristics ofB. juncea[9,14]。
Previous studies have shown that the HMA4 N-terminal and C-terminal domains can function separately and can interact [12]。The N-terminal domains that are responsible primarily for transport activity require energy in the form of ATP. Evidence for this fact was provided via an altered AtHMA4D401A protein that was substituted in place of the strictly conserved ATPase sequence DKTGT, which resulted in the disruption of metal tolerance by AtHMA4 [11]。However, the position and function of the C-terminal domain remain unclear. In addition to their binding activity, C-terminal domains may participate in subcellular localization, self-chaperone activity, metal concentration perception, and regulation of transport activity. Catherine et al. demonstrated that the expression of TcHMA4-C and AtHMA4-C provided Cd tolerance but that the tolerance provided by AtHMA4-C was much lower. In contrast, overexpression of the entireHMA4fromThlaspiorArabidopsisresulted in no significant difference in Cd sensitivity [12]。这些发现也暗示N-terminal and C-terminal domains of HMA4 could function separately and that HMA4 was subjected to positive selection during adaptive evolution, especially the C-terminal region.
BjHMA4 has a relatively large number of CC dipeptides (Cys pairs) because, as occurred for BjHMA4, BjHMA4R was similarly inserted between the first two CC pairs of AtHMA4, as shown in Fig.1.The first two CC pairs of AtHMA4 are indicated by blue numbers in Fig.1.The first two pairs are the only conserved part of the C-terminal region of the Cd/Pb transporter AtHMA3 [18]。Catherine et al. reported that these pairs might play an important role in metal transport. However, the substitutions AtHMA4C754/5A and AtHMA4C782/3A, which affect the first two Cys pairs within the C-terminal region of the protein, had no effect onycf1andzrc1complementation [11]。Interestingly, BjHMA4 does not present apparent transport and binding activity, but BjHMA4R exhibited strong biological activity in the present study, and we speculate that this region of HMA4 is responsible for heavy metal binding and may function as a sensor or buffer that interacts with other proteins and does not function as a self-chaperone that participates in self-transport activity [11,19]。
The mechanism of high Cd tolerance conferred byBjHMA4R
The yeast strains used in the present study had distinctly different genetic backgrounds: BY4741 is the WT;ycf1has a mutation in an ABC transporter (YCF1 [Yeast Cadmium Factor 1], which drives Cd-diglutathione complexes to the vacuole, is disrupted); andzrc1,cot1andzrc1&cot1(YK44) are mutants whose CDF cation transporters ZRC1 and/or COT1 are disrupted. Thus, the Cd tolerance phenotypes could not simply be interpreted as specific functional complementation of increased ion compartmentation, as BjHMA4R also alleviates the Cd sensitivity of WT yeast under high Cd concentrations. We speculated that the common mechanism by which BjHMA4R conferred Cd tolerance to yeast cells was attributed to strong binding activity in the cytosol. The subcellular localization of BjHMA4R in yeast supported this speculation.
In the present study, we observed reduced Cd accumulations in BjHMA4R-transformed YK44 andE. coli; the only explanation for this phenomenon is that the expression of BjHMA4R promoted Cd efflux. This could also improve the cells’ Cd tolerance to some extent, thus prompting the growth of yeast andE. coli.With respect to the mechanism, we speculated that BjHMA4R is a small peptide (115 amino acids) that could interact with other metal transporters as a chaperone such as ATX1 [20,21], resulting in increased Cd efflux. Only nonplant eukaryotes do not contain members of the P1B-ATPase family, soE. colihas HMA4 but yeast does not. We speculated that the P1B腺苷三磷酸酶家族在Cd acc导致更大的减少umulation inE. colithan in yeast. There was no significant difference in Zn accumulation between BjHMA4R-transformed yeast cells and BL21 or control cells under 200 μM ZnCl2stress, except in BjHMA4R-transformed wild-type yeast BY4741 cells, which showed slightly decreased Zn accumulation compared with control cells. These findings suggested that BjHMA4R might not bind Zn strongly under 200 μM ZnCl2stress and thus would not affect Zn homeostasis in cells.
Substrate specificity, form and binding kinetics of BjHMA4R
The expression profiles ofBjHMA4suggested that the most suitable substrates of BjHMA4 were Zn and Cd. The yeast drop-tests indicated that the substrates of BjHMA4R were Zn and Cd, andE. colidrop-tests verified this. None of theBjHMA4R-transformed yeast strains exhibited metal tolerance under high Ni, Co or Pb concentrations. However, all of the BjHMA4R-transformed yeast strains exhibited significant and similar levels of Cd tolerance, and theBjHMA4R-transformed YK44 cells exhibited Zn tolerance under 1.5 mM Zn stress.
Verret et al. showed that, in contrast to YCF1, AtHMA4 does not need a glutathione S-conjugated form of Cd for transportation [11]。This finding tentatively confirmed that the entire suite of AtHMA4 transport activity did not require the glutathione S-conjugated form in the in vivo experiment but could not entirely exclude the possibility that some other small molecules participate in the transportation. Additional experiments are needed to determine the substrate form of HMA4, and we will determine the substrate form of BjHMA4R via biochemical assays in vitro.
We believe that onlyBjHMA4R-transformed YK44 exhibited Zn tolerance under low Zn concentrations because of the relatively high Zn accumulation in the cytosol of YK44 cells. ZRC1 and COT1 are both localized in the vacuolar membranes of yeast, and the disruption of both transporters weakened the Zn compartmentation into the vacuoles. Under relatively high Zn concentrations (such as 4 mM), the growth ofBjHMA4R-transformed YK44 and control cells on solid media was completely inhibited; all of the other yeast strains (BY4741,ycf1,zrc1,cot1), regardless of whether expressedBjHMA4Ror not, grew well on solid media that contained with 4 mM Zn. This case was quite different from that of Cd stress, and we proposed that the binding kinetics of Zn and Cd varied greatly and that the affinity of BjHMA4R for Cd was much higher than that for Zn. Thus, theKmof BjHMA4R for Cd may be much lower than that for Zn, and theVmaxof BjHMA4R for Cd may be much higher than that for Zn. This speculation requires the biochemical characterization of BjHMA4R in vitro, and the results of ourE. coliexperiments supported the above speculation.
On the basis of our findings, we hypothesize that BjHMA4R functions as a sensor or buffer region in BjHMA4 and that its activity is regulated by metal concentration. Functional analysis of AtHMA4 and TcHMA4 by heterologous expression in yeast yielded controversial results. We proposed that the Cd concentration used in the yeast stress experiments was the key factor that caused the opposite results. With respect to AtHMA4, the highest Cd concentration used in the yeast stress experiments was 0.12 μM [7]; the lowest, 10 μM [12]。关于TcHMA4, Cd concentra最低tion used in the yeast stress experiments was 75 μM [14]; the highest, 50 μM [12]。From this perspective, we can explain the seemingly paradoxical result that Zn tolerance and Zn accumulation phenotypes occurred simultaneously in the yeast strain expressing AtHMA4 under very high Zn (20 mM) [11]。
The AhHMA4 C-terminus showed high Zn tolerance and low Cd tolerance. Partial peptides from the TcHMA4 C-terminus showed relatively high Cd tolerance (200 μM CdCl2, but the substrate specificity was not shown in this paper). BjHMA4R presented high Cd tolerance (500 and 600 μM CdCl2in yeast andE. coli, respectively) and low Zn tolerance under limited conditions (such as in the impaired-vacuole-compartmentation yeast YK44 and in the no-vacuoleE. coli), and BjHMA4R did not improve yeast orE. coliNi, Co or Pb tolerance. Thus, the substrates of BjHMA4R may only be Cd and Zn, but it has high affinity with Cd. Moreover, considering that orthologs of BjHMA4 have a highly conserved N-terminus and more divergent C-terminus and homologous sequences to BjHMA4R only exist in Brassicaceae, we suggest that domains of the HMA4 C-terminus may play important roles in determining substrate specificity and binding affinity.
Application prospects of BjHMA4R
BjHMA4R may have useful applications with respect to the treatment of wastewater that contains Cd by microbiological methods and enhancing the phytoremediation potential of plants via biotechnology. We are currently expressingBjHMA4RinArabidopsis和水稻研究其潜在的城市ntal applications; our efforts include the development of plants that exhibit increased heavy metal tolerance and possibly increased hyperaccumulation of metals. Furthermore, we are currently expressing BjHMA4R in human cells to determine whether BjHMA4R participates in the control of oxidative stress components such as metallothionein (MT).
有限公司nclusions
We clonedBjHMA4fromBrassica juncea.The deduced amino acid sequence contains a four-repeat region, named BjHMA4R. BjHMA4R has 4 CC pairs that may be involved in heavy metal binding. Detailed characterization of BjHMA4R indicated that BjHMA4R localized in the yeast cytosol and substantially and specifically improved Cd tolerance and accumulation under low heavy metal concentrations.E. coliexperiments verified this function. Thus, our study found a candidate gene that could be applied to the treatment of wastewater that contains Cd by microbiological methods and phytoremediation.
Methods
Experiments onBjHMA4
Plant materials
Seeds of Indian mustard (B. junceaL.) were kindly provided by the National Germplasm Resources Lab, USA (IP: 173874). SterilizedB. junceal .种子发芽在Murashige和斯库(MS) solid medium at 25 °C under a 16-h photoperiod. Seven days after germination, the seedlings were transferred to half-strength Hoagland solution, which was refreshed every 5 days.
The rice and wheat plants and culture conditons were identical to Tan et al. [22]。
Gene cloning
The total RNA was extracted from the Indian mustard seedlings using TRIzol reagent (Invitrogen, San Diego, CA). cDNA was synthesized from the DNase-treated total RNA using a GIBCO BRL SuperScript First-Strand Synthesis System. The full-length coding sequence was isolated in three stages. An oligonucleotide primer designed from the most conserved 3` regions ofHMA2/4cDNAs fromA. thaliana,T. caerulescensandA. halleri(BjHMA4–3`: 5`-TGACATGAAACTGAAAGGTGGTTC-3`) was used for 3` rapid amplification of cDNA ends (3’RACE) (SMART RACE kit, Clontech); the 3` cDNA was sequenced and used to design another primer (BjHMA4-interR: 5`-ACAACCTGAACCACCTTT-3`). Similarly, an oligonucleotide primer was designed from the most conserved 5` regions ofHMA2/4cDNAs fromA. thaliana,T. caerulescensandA. halleri(BjHMA4-interF: 5`-AAGAGTTACTTCGATGTTTT-3`), after which a large partial cDNA fragment of BjHMA4 was cloned using the primers BjHMA4-interF and BjHMA4-interR. BjHMA4-specific primers designed from the above partial cDNA (BjHMA4–5`1: 5`-TCAGACGGACAACAGATT-3` and BjHMA4–5`2: 5`-CAACAGATTCCCAAAACA-3`) were used for 5’RACE (SMART RACE kit, Clontech). Last, full-length cDNA was cloned using BjHMA4-F: 5`-ACGACGCCGGACTCAAGGA-3` and BjHMA4-R: 5`-GCGTCTGAGAATCAAAGC-3` primers. The PCR products were then purified and cloned into a pMD18-T vector (Takara Bio), after which sequencing was performed.
Sequence analysis
HMA4 sequences were aligned using ClustalW [23] and DNAMAN 6.0; the alignment was then manually adjusted using PSBLAST pairwise alignments. Putative transmembrane domains were identified using TMHMM [24] and hydropathy analyses [25]。
Stress treatments and quantitative real-time PCR analysis
Hydroponic Indian mustard seedlings were used for detectingBjHMA4expression in various tissues at different stages. The roots of seedlings treated with 600 μM ZnCl2for different durations were used for measuringBjHMA4expression under Zn stress. Seedlings treated with CdCl2(200 μM), ZnCl2(2000 μM), or NiCl2(400 μM) for 6 h and seedlings for which Zn, Mn and Cu supplies were absent for 1 week were used to measureBjHMA4expression levels in the roots and shoots under various abiotic stresses.
Real-time PCR was performed using SYBR Green (Toyobo, Osaka, Japan) with an MX3000P instrument (Stratagene Laboratories, La Jolla, CA). TheBjHMA4primers used for real-time PCR were 5′-TCTGTGGCAAAGAAGTAA-3′ and 5′-ACCAAACTAGACGACCCT-3′, and the endogenous reference primers were those associated with theB. junceaactin gene (GenBank ID: KM881428.1): 5′-AATCGCTGACCGTATGAG-3′ and 5′-TCTGTTGGAAGGTGCTGA-3′.
Yeast transformation and Zn/cd tolerance analysis
The methods were identical to Tan et al. [22]。
Plant plasmid construction
The methods were similar to Tan et al. [22]。The pUN1301-BjHMA4 and pLI-Act-MCS L42 rev-BjHMA4 expression constructs were generated by inserting BjHMA4 expression cassettes into the BamHI/KpnI and PstI/HindIII sites of pUN1301 (driven by the ubiquitin promoter) and pLI-Act-MCS L42 rev (driven by the rice Actin1 promoter), respectively.
Plant transformation
The methods were identical to Tan et al. [22]。
Metal contents and translocation analysis
The methods were identical to Tan et al. [22]。
Experiments on BjHMA4R
Yeast andEscherichia colicultures and transformation
TheSaccharomyces cerevisiaereference strain BY4741 (MATαhis3D1 leu2D0 met15D0 ura3D0) and mutant strainszrc1(similar to BY4741 but containingYMR243c: kanMX4),ycf1(similar to BY4741 but containingYDR135c::kanMX4),cot1(strain BY4742:MATαhis3D1 leu2D0 lys2D0 ura3D0 YOR316c::kanMX4) andend3(similar to BY4741 but containing YNL084c::kanMX4) were obtained from Euroscarf (Frankfurt, Germany). Prof. Dietrich H. Nies (Martin Luther University, Germany) kindly provided the yeast mutant strain YK44 (ura3–52 his3–200, ∆zrc1, ∆cot1,MATα). The cells were grown in YPD (1% yeast/2% peptone/2% dextrose; Difco), YPGAL (1% yeast/2% peptone/2% galactose; Difco), and SD (Difco; Synthetic medium with 2% dextrose) media. TheE. colistrain BL21 was part of a commercial batch from Transgen Biotech, China. LB medium (1% tryptone/0.5% yeast extract/1% NaCl; protein expression was induced by 0.5 mM isopropyl β-D-1-thiogalactopyranoside [IPTG]) was used for growingE. coli.
The repeat region ofBjHMA4cDNA (BjHMA4R) was obtained using BjHMA4R-specific primers (BjHMA4R-F-KpnI: 5`-GGGGTACCATGAAGAAACCAAGTAGT-3` and BjHMA4R-R-XhoI: 5`-CCGCTCGAGTCAAGCAGTCCCCACATG-3`), and pMD18-BjHMA4 served as a template. TheBjHMA4RcDNAs were cloned into a pYES2 vector (Invitrogen) and a pEASY-Blunt E1 expression vector (Transgen Biotech, China), which were subsequently transformed into yeast andE. coli, respectively, using chemical methods [26,27]。
Subcellular localization
A pUG36 expression vector (provided by Prof. Filleur), which harbors the eGFP-BjHMA4R fusion protein, was used to observe the localization of BjHMA4R in yeast. BjHMA4R cDNA was amplified using BjHMA4R-specific primers (gfpBjHMA4R-F-XbaI: 5`-TGCTCTAGAATGAAGAAACCAAGTAGT-3`; BjHMA4R-R-Xho我:5“-CCGCTCGAGTCAAGCAGTCCCCACATG-3”)。的resulting PCR products were cloned into the XbaI-XhoI restriction sites of pUG36 for expression in yeast. The yeast strains transformed with pUG36/BjHMA4R or pUG36 were grown in liquid SD-URA/glucose (Glu) media (with or without methionine [Met]) for induction until the stationary phase. Yeast vacuolar membranes were selectively stained with FM4–64 (Catalog #: 70021, Biotium), a red fluorescent probe that has been demonstrated to selectively stain yeast vacuolar membranes [28]。The cells (1000 μL at an OD6000.4 - -0.6)的孵化10μL fm4 - 64 (2 mg/mL) for 20 min at 29 °C, washed twice with YPD medium, incubated with shaking for 2 h in 3 mL YPD and then washed twice with SD-URA medium before observations via confocal microscopy (Zeiss LSM880). For visualization of eGFP fluorescence, excitation at 488 nm was performed with an argon ion laser, and emission signals between 500 and 535 nm were collected. For visualization of eGFP and FM4–64 colocalization, a second detector was used to collect emission signals from 650 to 800 nm [29]。
Metal tolerance tests
Yeast cells were cultured in YPD at 30 °C overnight with shaking. We used 5-μL aliquots of yeast cultures that had reached an OD600of 1 to produce three tenfold serial dilutions. We used the same sterilized media for drop-test experiments on solid media. Metal solutions (ZnCl2, CdCl2, Pb(NO3)2, Ni(NO3)2and CoCl2) were added to the liquid or solid media to produce different concentrations, as indicated in the figure legends. All drop-test experiments were performed in duplicate and were independently repeated at least three times.E. colicells were assayed for heavy metal tolerance via LB media supplemented with ZnCl2and CdCl2.
Yeast andE. colicells were cultured in liquid YPD and LB media that contained CdCl2.The cells within 15 mL of a bacteria and yeast fluid were collected by centrifugation at two different time points after treatment, after which the cells were dried at 95 °C for 48 h to determine their dry weight.
Determination of intracellular metal contents
Yeast strains were grown overnight at 30 °C in liquid YPD medium. The cells were incubated at a density adjusted to OD600 = 0.2 in the presence of 30 μM CdCl2and 200 μM ZnCl2for different durations. After incubation, the cells collected by centrifugation were washed twice with 50 mM EDTA followed by once with distilled water, after which they were dried at 95 °C for 48 h to determine their metal contents.
Cells resuspended in distilled water were mixed with an equal volume of concentrated HNO3, incubated at 95 °C for 2 h and then diluted with 2 volumes of distilled water. The Cd content in the cells was determined with an inductively coupled plasma trace analyzer emission spectrometer (Thermo ICAP-Q).
A similar procedure was carried out to determine the Cd and Zn accumulation inE. colicells transformed with BjHMA4R clones. All the reported metal accumulation values are the means ± SEs, as determined via four replicate experiments.
Measurement of intracellular Cd2+flux in response to 30 μM CdCl2stress
Net Cd2+flux was measured using the non-invasive Micro-test Technology (NMT). The samples were measured in the testing solution with 30 μM Cd2+after the electrodes were calibrated in two Cd2+concentrations (solution I, 10 μM; solution II, 100 μM). The detailed methods were described previously [30,31]。
Statistical analysis
Statistical analysis was performed using the statistical software package SPSS 19.0 (SPSS Science, Chicago, IL). A two-tailedt-test was conducted to analyze various indexes of the transgenic plants compared with the WT plants at 5 and 1% levels of probability. The experiments were repeated three times. The data are expressed as the means ± SD.
Statistical analysis was performed by one-way ANOVA using the statistical software package SPSS 19.0. Following ANOVA, Duncan’s multiple-range testwas performed to make comparisons between treatments [32]。
Abbreviations
- Cd:
-
Cadmium
- 有限公司:
-
有限公司balt
- HMA:
-
Heavy metal ATPase
- NMT:
-
Non-invasive Micro-test Technology
- Pb:
-
Lead
- QTL:
-
Quantitative trait locus
- WT:
-
Wild-type
- Zn:
-
Zinc
References
Nagahara U. Genome analysis in Brassica with special reference to the experimental formation ofB. napusand peculiar mode of fertilisation. Jpn J Bot. 1935;7:389–452.
Kim CK, Seol YJ, Perumal S, Lee J, Waminal NE, Jayakodi M, et al. Re-exploration of U’s triangle Brassica species based on chloroplast genomes and 45S nrDNA sequences. Sci Rep. 2018;8:7353.
Anjum NA, et al., editors. The plant family Brassicaceae contribution towards phytoremediation. Dordrecht: Springer; 2012. ISBN 9789400739130
Schneider T, Haag-Kerwer A, Maetz M, Niecke M, Povh B, Rausch T, et al. Micro-PIXE studies of elemental distribution in cd-accumulatingBrassica junceaL. Nucl Instrum Methods Phys Res, Sect B. 1999;158:329–34.
Pantola R. Correlation between copper induced changes in peroxidase activities and its accumulation in seedling roots ofBrassica juncea.Mycopathologia. 2013;11:59–64.
Su Y, Sridhar BBM, Han FX, Diehl SV, Monts DL. Effect of bioaccumulation of Cs and Sr natural isotopes on foliar structure and plant spectral reflectance of indian mustard (Brassica juncea).Water Air Soil Pollut. 2007;180:65–74.
Axelsen KB, Palmgren MG. Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 2001;126:696–706.
有限公司bbett CS, Hussain D, Haydon MJ. Structural and functional relationships between type 1B heavy metal-transporting P-type ATPases in Arabidopsis. New Phytol. 2003;159:315–21.
Hussain D, Haydon MJ, Wang Y, Wong E, Sherson SM, et al. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell. 2004;16:1327–39.
Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol. 1998;46:8–101.
Verret F, Gravot A, Auroy P, Preveral S, Forestier C, Vavasseur A, et al. Heavy metal transport by AtHMA4 involves the N-terminal degenerated metal binding domain and the C-terminal his11 stretch. FEBS Lett. 2005;579:1515–22.
Catherine B, Nancy R, Pierre C, Michel L, Nathalie V. A novel CPx-ATPase from the cadmium hyperaccumulatorThlaspi caerulescens.FEBS Lett. 2004;569:140–8.
Mills RF, Krijger GC, Baccarini PJ, Hall JL, Williams LE. Functional expression of AtHMA4, a P1B-typeATPase of the Zn/Co/Cd/Pb subclass. Plant J. 2003;35:164–76.
Papoyan A, Kochian LV. Identification ofThlaspi caerulescensgenes that may be involved in heavy metal hyperaccumulation and tolerance. Characterization of a novel heavy metal transporting ATPase. Plant Physiol. 2004;136:3814–23.
Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, et al. Evolution of metal hyperaccumulation requiredcis-regulatory changes and triplication of HMA4. Nature. 2008;453:391–5.
Williams LE, Mills RF. P1B-ATPases – an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 2005;10:491–502.
Li L, Kaplan J. Defects in the yeast high affinity iron transport system result in increased metal sensitivity because of the increased expression of transporters with a broad transition metal specificity. J Biol Chem. 1998;273:22181–7.
Gravot A, Lieutaud A, Verret F, Auroy P, Vavasseur A, Richaud P. AtHMA3, a plant P1B-ATPase, functions as a cd/Pb transporter in yeast. FEBS Lett. 2004;561:22–8.
Battistoni A, Pacello F, Mazzetti AP, Capo C, Kroll JS, Langford PR, et al. A histidine-rich metal binding domain at the N terminus of cu, Zn-superoxide dismutases from pathogenic bacteria: a novel strategy for metal chaperoning. J Biol Chem. 2001;276:30315–25.
Andrés-Colás N, Sancenón V, Rodríguez-Navarro S, Mayo S, Thiele DJ, Ecker JR, et al. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006;45:225–36.
Zhang YY, Chen K, Zhao FJ, Sun CJ, Jin C, Shi YH, et al. OsATX1 interacts with heavy metal P1B-type ATPases and affects copper transport and distribution. Plant Physiol. 2018;178:329–44.
Tan JJ, Wang JW, Chai TY, Zhang YX, Feng SS, Li Y, et al. Functional analyses ofTaHMA2, a P1B-type ATPase in wheat. Plant Biotechnol J. 2013;11:420–31.
Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80.
Krogh A, Larsson B, Heijne GV, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden markov model: application to complete genomes. J Mol Biol. 2001;305:567–80.
Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–32.
Gietz RD. High efficiency transformation with lithium acetate. In: Molecular Genetics of Yeast A Practical Approach; 1994.
Hanahan D, Jessee J, Bloom FR. Plasmid transformation ofEscherichia coliand other bacteria. Methods Enzymol. 1991;204:63–113.
Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–92.
有限公司urbot M, Willems G, Motte P, Arvidsson S, Roosens N, Saumitou-Laprade P, et al. A major quantitative trait locus for cadmium tolerance in Arabidopsis halleri colocalizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiol. 2007;144:1052–65.
Li S, Pan XX, Berry JO, Wang Y, Naren MS, et al. OsSEC24, a functional SEC24-like protein in rice, improves tolerance to iron deficiency and highpH by enhancing H+secretion mediated by PM-H+-ATPase. Plant Sci. 2015;233:61–71.
Yang G, Ma F, Wang Y, Feng CG, Li P, Xu Y, et al. Vesicle-related OsSEC27P enhances H+secretion in the iron deficient transgenic tobacco root. Chin Sci Bull. 2010;55:3298–304.
Duncan DB. Multiple range and multiple F-tests. Biometrics. 1955;11:1–42.
Acknowledgments
We thank Prof. Sophie Filleur (Universite´ Paris 7-Denis Diderot, UFR Sciences du Vivant) for providing us with pUG36 plasmids; Euroscarf (Frankfurt, Germany) for providing the yeast strainsycf1,zrc1,cot1andend3; and Prof. Dietrich H. Nies (Marin Luther University) for donating yeast strain YK44.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.: 31500201) and Project of Shaanxi Provincial Education Department (15JK1121). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Not applicable.
Author information
Affiliations
有限公司ntributions
JWW and TYC conceived and designed the research; JWW performed most of the experiments; SL WWX FRK and HPD provided technical assistance to JWW; JWW, SL, YZD and TYC analyzed the data and wrote the manuscript. TYC agrees to serve as the author responsible for contact and ensures communication. All authors read and approved the final manuscript.
有限公司rresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
有限公司nsent for publication
Not applicable.
有限公司mpeting interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Data S1.Semiquantitative RT-PCR analysis of transgenic rice and wheat plants.a,c: Lane WT = an untransformed plant; lane number shows semiquantitative RT-PCR analysis of different transgenic plants; P, positive control.b,d: Real-time PCR analysis ofBjHMA4in the transgenic rice and wheat. WT = an untransformed plant. Os1, Os2, and Os3 and Ta1, Ta2, and Ta3 show 3 independent transgenic rice and wheat lines, respectively. The data are expressed as the mean ± SE of three replicates; * and ** indicate significant levels at 5 and 1% (evaluated by Student’sttest), respectively. (JPG 216 kb)
Additional file 2:
Data S2.Sequence alignment of AhHMA4, AtHMA4 and TcHMA4 (cited from Courbot et al. and modified). Asterisks (*) indicate the highly conserved amino acid residues in Brassicaceae. The start of the C-terminal fragment from AhHMA4 used in the yeast tolerance assay is indicated with a bracket [29]。The 384- (underlined) and 141-amino acid (shaded in blue) partial peptides from the C-terminus of TcHMA4 used in the yeast tolerance assay. (JPG 432 kb)
Additional file 3:
Data S3.Growth of yeast cells expressingBjHMA4Runder Ni, Co, Pb stress. BY4741, YK44,ycf1,cot1andzrc1transformants expressed pYES2 (negative control) andBjHMA4R, respectively. The cultures were adjusted to an OD600of 1 and were serially diluted 10-fold in water. Five-microliter aliquots of each dilution were spotted either on nonselective YPD plates or on YPD plates supplemented with 5 mM Ni(NO3)2, 5 mM Co(NO3)2and 10 mM Pb(NO3)2.经过3天的孵化30°C,这些盘子were imaged. The dilutions are indicated in the above figure, and three individual clones of each yeast transformant were analyzed. (JPG 10974 kb)
Additional file 4:
Data S4.Ni, Co and Pb contents of yeast andE. coliexpressingBjHMA4R.a, b, d, e, g, h:Yeast BY4741 and YK44 cells transformed with a pYES2 plasmid or a pYES2 plasmid that harboredBjHMA4Rwere grown in normal liquid YPD medium overnight. Then, they were supplemented with 200 μM Ni(NO3)2, Co(NO3)2and Pb(NO3)2.The cells were incubated at 30 °C for 30 min, 70 min, 5 h, 10 h or 21 h.c、f,我:E. coliBL21 cells transformed with a pEASY-Blunt E1 expression plasmid or a pEASY-Blunt E1 expression plasmid that harboredBjHMA4Rwere grown in normal liquid LB medium overnight, then supplemented with 200 μM Ni(NO3)2, Co(NO3)2and Pb(NO3)2.The cells were incubated at 37 °C for 10 h and 21 h. The metal contents of the samples were analyzed with inductively coupled plasma-mass spectrometry (ICP-MS). The results are the means ± SEs of four independent experiments performed with four different colonies. Different letters above the columns indicate significant differences (P < 0.05) between cell groups under the same stress treatment. (JPG 637 kb)
Rights and permissions
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, J., Liang, S., Xiang, W.et al.A repeat region from theBrassica juncea HMA4geneBjHMA4Ris specifically involved in Cd2+binding in the cytosol under low heavy metal concentrations.BMC Plant Biol19,89 (2019). https://doi.org/10.1186/s12870-019-1674-5
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/s12870-019-1674-5
Keywords
- Heavy metal ATPase (HMA)
- Heavy metal tolerance
- Cadmium
- Zinc
- Substrate specificity
- Hyperaccumulation