- Research article
- Open Access
- Published:
Co-expression of SpSOS1 and SpAHA1 in transgenicArabidopsisplants improves salinity tolerance
BMCPlant Biologyvolume19, Article number:74(2019)
Abstract
Background
Na+extrusion from cells is important for plant growth in high saline environments. SOS1 (salt overly sensitive 1), an Na+/H+antiporter located in the plasma membrane (PM), functions in toxic Na+extrusion from cells using energy from an electrochemical proton gradient produced by a PM-localized H+-ATPase (AHA). Therefore, SOS1 and AHA are involved in plant adaption to salt stress.
Results
In this study, the genes encoding SOS1 and AHA from the halophyteSesuvium portulacastrum(SpSOS1andSpAHA1, respectively) were introduced together or singly intoArabidopsisplants. The results indicated that either SpSOS1 or SpAHA1 conferred salt tolerance to transgenic plants and, as expected,Arabidopsisplants expressing bothSpSOS1andSpAHA1grew better under salt stress than plants expressing onlySpSOS1orSpAHA1. In response to NaCl treatment, Na+and H+in the roots of plants transformed withSpSOS1orSpAHA1effluxed faster than wild-type (WT) plant roots. Furthermore, roots co-expressingSpSOS1andSpAHA1had higher Na+and H+efflux rates than singleSpSOS1/SpAHA1-expressing transgenic plants, resulting in the former amassing less Na+than the latter. As seen from comparative analyses of plants exposed to salinity stress, the malondialdehyde (MDA) content was lowest in the co-transgenicSpSOS1andSpAHA1plants, but the K+level was the highest.
Conclusion
These results suggest SpSOS1 and SpAHA1 coordinate to alleviate salt toxicity by increasing the efficiency of Na+extrusion to maintain K+homeostasis and protect the PM from oxidative damage induced by salt stress.
Background
盐,尤其是生理盐水,可以有毒植物through inhibition of important biochemical and physiological processes, such as protein synthesis, photosynthesis, and enzymatic reactions, after moving into the cytosol from soils [1].While salt stress can inhibit plant growth and development, many types of plants are able to grow in high salinity environments because they have complex mechanisms that facilitate adaptation to salinity stress [2].Of these mechanisms, the ability to transport excess Na+out of cells is critical to salt tolerance. SOS1 (salt overly sensitive 1) is a Na+/H+antiporter and the only Na+efflux protein present in plant plasma membranes (PMs) characterized to date. SOS1 mediates extrusion of Na+through a proton gradient generated by the H+-ATPase (AHA) in the PM [3].Therefore, SOS1 and AHA are two key plant halotolerance factors.
PM H+-ATPase is encoded by a large family of genes [4,5].Bioinformatics analyses ofArabidopsis and genomic sequences of rice revealed the presence of 11 and 10 PM AHAs, respectively [6,7].Of these AHAs, NaCl treatment induced expression of three, AtAHA1, AtAHA2,andAtAHA3, inArabidopsis[8].The transcript levels of PM AHA were found to be higher in a salt-tolerant poplar than a salt-sensitive poplar [9].In addition, PM AHA mRNA is more abundant in halophytes than glycophytes [10,11].Salinity causes upregulation of PMAHAgene expression, as well as accelerates protein biosynthesis and H+-pumping activity in some plants [12,13,14].AHA in a salt-tolerant rice species has higher activity than in a salt-sensitive rice species [15].AnArabidopsisPM AHA4 mutant has dramatically reduced growth when exposed to salt stress compared to WT [16].Expression of a constitutively activated PM AHA lacking the autoinhibitory domain in transgenic tobacco plants increases salt tolerance compared to untransformed plants [17].
SOS1genes have been found in many plants [18,19,20,21,22,23,24,25].Of these,ArabidopsisSOS1 (AtSOS1) was the first PM Na+/H+antiporter to be thoroughly physiologically, biochemically, and molecularly characterized [18,26].Exposure to salinity stress increasesSOS1transcript abundance in wheat plants [19], induces the accumulation ofSOS1mRNA in rice plants [27], and causes upregulation ofSOS1transcription inArabidopsis[28].Under high salt conditions,SOS1mRNA levels are higher inThellungiella salsuginea(a halophyticArabidopsis-relative plant) thanArabidopsis[20].MutantArabidopsisplants lacking SOS1 are extremely sensitive to salt stress [18,29].Thellungiella salsuginealines expressing SOS1-RNAi (RNA interference) are sensitive to salt [20].The salt sensitivity of anArabidopsis sos1mutant can be overcome by transforming in native or other plantSOS1genes [27,28].ArabidopsisoverexpressingAtSOS1is more salt tolerant than WT plants [30].Expression of wheat SOS1 (TaSOS1) in transgenic tobacco plants improves their growth following NaCl treatment [31].SOS1 uses the proton gradient established by PM AHA to exchange Na+for H+across the PM [3,27].The aforementioned data indicate the PM Na+/H+antiporter SOS1 and H+-ATPase AHA are involved in plant salt tolerance, where an Na+/H+antiporter utilizes the proton gradient generated by H+-ATPase to move Na+from the cytoplasm to the external medium and help plant cells maintain non-toxic cytosolic concentrations of Na+. Therefore, theoretically, coordinating SOS1 and AHA could enhance Na+extrusion, where co-expression of these two genes should confer better tolerance to salinity to transgenic plants. However, it has not been reported whether SOS1 and AHA1 function cooperatively in transgenic plants to more efficiently improve salinity tolerance.
Sesuvium portulacastrumis a halophyte that grows optimally in the presence of 200–300 mM NaCl [32].When growing in a saline environment,S. portulacastrumcells accumulate large amounts of Na+despite salt glands and bladders not being present in all tissues [33,34,35], suggestingS. portulacastrummay have a unique ability to remove Na+from cells. The SOS1 protein functions as a PM Na+/H+antiporter driven by the proton gradient that is produced by the PM H+-ATPase AHA, so they are considered as superior salt tolerance determinants [3,36].TheSpAHA1andSpSOS1genes encode a PM H+-ATPase and Na+/H+antiporter, respectively, and are more highly transcribed inS. portulacastrumplants exposed to salt stress. SpSOS1 more efficiently mediates Na+removal using a proton gradient created by SpAHA1 inSpAHA1-SpSOS1co-transgenic yeast cells, where yeast cells co-expressingSpSOS1andSpAHA1grow better following NaCl treatment than cells transformed with onlySpSOS1orSpAHA1[3].Over-expression ofSpAHA1conferred salt tolerance to transgenicArabidopsis[37].SpSOS1 complemented the salt sensitivity of transgenicArabidopsis sos1mutant plants [38].These results suggest that SpSOS1 and SpAHA1 are involved in salt tolerance ofS. portulacastrum, and co-expression ofSpAHA1andSpSOS1may improve transgenic plant salt tolerance. To test this hypothesis,SpAHA1andSpSOS1genes were co-transformed intoArabidopsisplants. Functional analyses indicate thatArabidopsisplants co-expressingSpSOS1andSpAHA1had better salt tolerance than plants expressing either gene alone due to efficient Na+removal mediated by SpSOS1 using the extra proton gradient generated by SpAHA1. Therefore, genetic evidence may significantly guide development of more salt tolerant crops using PM-localized Na+/H+antiporters and H+-ATPases.
Results
Transgenic plant identification
SpSOS1andSpAHA1were transformed alone or together intoArabidopsisplants usingAgrobacteriacarrying pCAMBIA1304-SpSOS1, pCAMBIA1304-SpAHA1,or pCAMBIA1304-SpSOS1-SpAHA1. PCR analyses of genomic DNA performed usingSpAHA1/SpSOS1andhygBgene-specific primers revealed 12SpSOS1-, 11SpAHA1-, and 10SpSOS1-SpAHA1-transgenic lines were obtained (Additional file1: Figure S1).Total RNA was isolated from the above transgenic plant lines and RT-PCR analyses were used to study theSpAHA1andSpSOS1expression levels. TheSpAHA1gene was significantly expressed in all singleSpAHA1-transgenic lines, except for SpAHA1- lines 5 and 8. Of theSpSOS1-expressing single transgenic plants,SpSOS1-line 1 had the highestSpSOS1expression of theSpSOS1-transgenic lines. InSpAHA-SpSOS1co-expressing plants, the clearest expression of bothSpAHA1andSpSOS1在10号线(额外的文件吗2: Figure S2). Therefore, the T3 generation transgenic plants of the homozygousSpSOS1-line 1,SpAHA1-line 1, andSpAHA1-SpSOS1-line 10 were used to characterize the functions of SpSOS1 and SpAHA1.
SpSOS1 and SpAHA1 functioned together to more efficiently improve transgenic plant salt tolerance
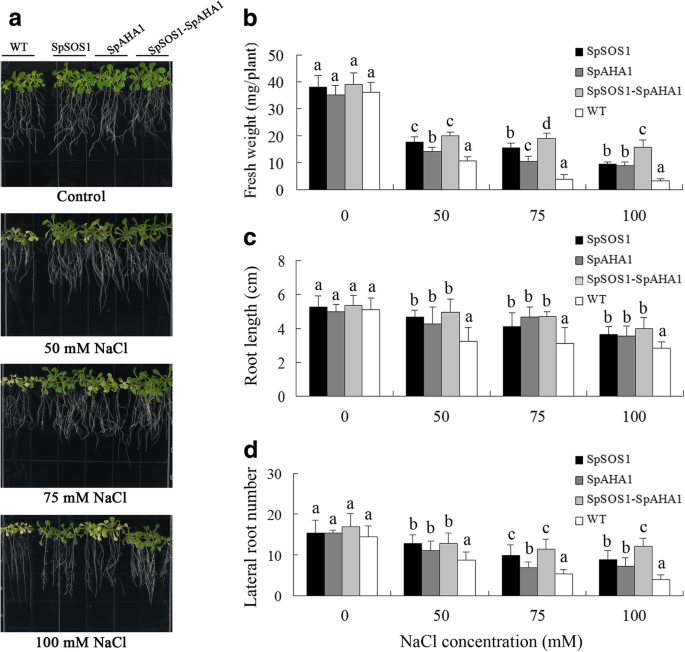
In plant cells, the PM Na+/H+antiporter SOS1 mediates Na+excretion using a proton gradient created by PM H+- - - - - - atp酶. Therefore, both of these proteins are involved in plant salt tolerance. Much evidence indicates that overexpressingSOS1orAHAincreases the salt tolerance of transgenic plants [39].In addition, our recent investigation found SpSOS1 and SpAHA1 function cooperatively in transgenic yeast cells, where yeast cells co-expressingSpSOS1andSpAHA1are better growers than cells transformed with onlySpAHA1orSpSOS1[3].Therefore, we hypothesized co-expression ofSpSOS1andSpAHA1would increase the salt tolerance of transgenic plants compared to plants transformed with onlySpSOS1orSpAHA1. To examine the influence ofSpSOS1-SpAHA1co-expression on the salt tolerance of transgenic plants, 5-day-oldArabidopsisWT,SpSOS1-expressing,SpAHA1-expressing, andSpSOS1-SpAHA1co-expressing seedlings were grown on MS plates containing 0, 50, 75, or 100 mM NaCl. Two weeks post-NaCl treatment, the seedlings were photographed and their fresh weight, root length, and lateral root number were measured.Upon exposure to salinity stress, the growth of all tested plants decreased, but expression of eitherSpSOS1orSpAHA1ameliorated this growth inhibition from NaCl treatment compared to WT plants. Furthermore, among all the transgenic plants, salt tolerance improved the most in plants co-expressingSpSOS1andSpAHA1based on growth in MS medium containing different concentrations of NaCl (Fig.1).
Growth of transgenic and WT seedlings under salt stress. Five-day-old seedlings grown on MS plates were transferred to MS plates containing 0, 50, 75, and 100 mM NaCl. a The seedlings were photographed after 2 weeks of growth. The growth was assessed based on fresh weight (b), root length (c), and number of lateral roots (d). Data are presented as mean ± SE of 12 replicates, where the different letters above the columns indicate statistically significant differences at ap < 0.05 level between the experimental cohorts. SpSOS1,SpSOS1-overexpressing plants; SpAHA1,SpAHA1-overexpressing plants; SpSOS1-SpAHA1,SpSOS1andSpAHA1co-expressing plants; WT, wild-type plants
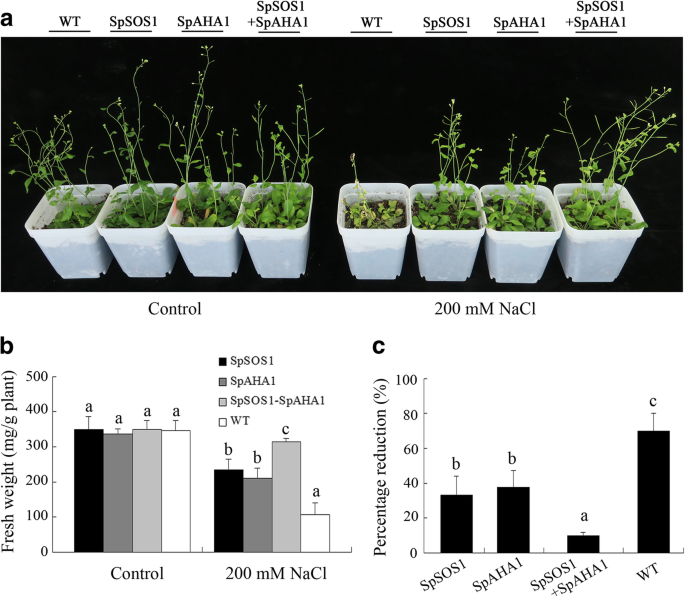
Similarly, the growth of transgenic and WT plants was inhibited in soil supplemented with 200 mM NaCl. However,Arabidopsisplants expressing bothSpSOS1andSpAHA1grew the best among the different experimental cohorts under these conditions (Fig.2a).SpAHA1-SpSOS1-line 10 displayed 26, 33, and 67% greater fresh weights thanSpSOS1-line 1,SpAHA1-line 1, and WT plants, respectively (Fig.2b). The percent reduction in growth of plant lines treated with NaCl was ordered:SpSOS1-SpAHA1co-expressing plants <SpSOS1-expressing plants<SpAHA1-expressing plants
Growth of transgenic and WT seedlings. Seven-day-old WT and transgenic seedlings were transferred from MS plates into soil (4 plants/pot) grown for 4 weeks. The plants were then treated with 0 or 200 mM NaCl. Ten days post-treatment, the plants were photographed (a) and their fresh weight was measured (b). The two preparations were performed at different times to more accurately assess the salt tolerance of the transgenic plants and (c) the relative change (percentage reduction) in fresh weight in the presence of salt stress relative to the nonstressed control was determined. Data are presented as mean ± SE of nine replicates. Different letters above the columns indicate statistically significant differences at ap < 0.05 level among the different experimental cohorts. SpSOS1,SpSOS1-overexpressing plants; SpAHA1,SpAHA1-overexpressing plants; SpSOS1 + SpAHA1,SpSOS1andSpAHA1co-expressing plants; WT, wild-type plants
SpSOS1-SpAHA1co-expressingArabidopsisplants had higher H+efflux rates thanSpSOS1-orSpAHA1-expressing plants under high saline conditions
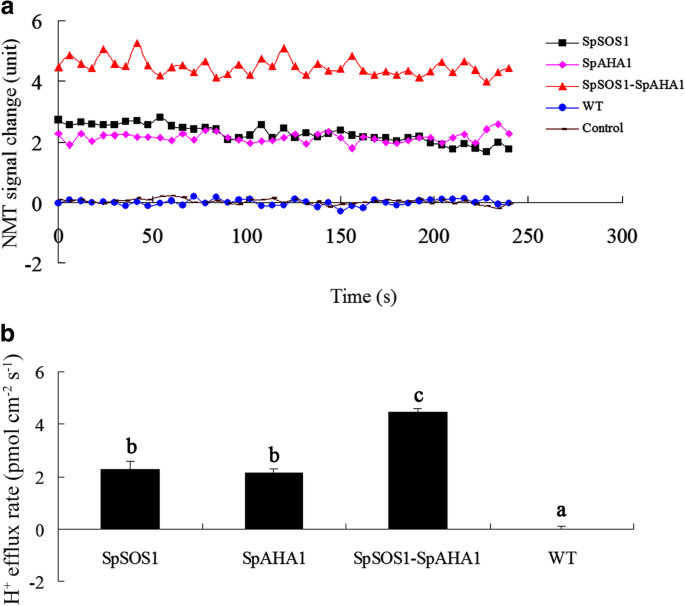
Net H+flux at the roots of WT plants was close to the mock control (Fig.3A), which is in full agreement with the recent report that both transient and long-term salinity exposure did not induce H+efflux fromArabidopsisroots [8].These results suggested that H+efflux might be balanced by H+influx at the roots exposed to salinity stress. PM H+-ATPase activity is a major factor in H+excretion at the PM [40].It was recently reported that SpAHA1 can function as an H+-ATPase on vesicles isolated from yeast cells expressingSpAHA1[3].Roots expressing SpAHA1 had a faster net H+efflux than the WT plants under saline conditions, suggesting SpAHA1 is responsible for the extra H+efflux, i.e., SpAHA1 pumped more protons out of the cells. It is not expected that protons were extruded faster in roots transformed withSpSOS1relative to WT plants and the phenomenon might be from feedback regulation of Na+extrusion mediated by SpSOS1. This hypothesis is also supported by the H+flux in the roots ofSpSOS1-SpAHA1co-expressing transgenic plants (Fig.3) being the highest among all the transgenic plants, where the H+efflux rates in the roots co-expressingSpSOS1-SpAHA1were 49 and 52% greater thanSpSOS1- andSpAHA1-expressing roots, respectively.
H+flux in roots of NaCl-treatedArabidopsisplants. Seedlings were grown for 3 days on MS plates supplemented with 100 mM NaCl and then H+flux in the roots was measured using the non-invasive micro-test technology (NMT) technique described in the Methods section. (a) Changes in the NMT signals are expressed as arbitrary units. (b) H+flux is expressed as the amount of efflux per second per square centimeter (pmol•cm− 2•s− 1). Data are presented as mean ± SE of six replicates. Different letters above the columns indicate statistically significant differences at ap < 0.05 level among the different experimental cohorts. SpSOS1,SpSOS1-overexpressing plants; SpAHA1,SpAHA1-overexpressing plants; SpSOS1-SpAHA1,SpSOS1andSpAHA1co-expressing plants; WT, wild-type plants; Control, mock controls
Plants co-expressingSpSOS1-SpAHA1had higher Na+efflux in roots and less Na+accumulation after NaCl treatment
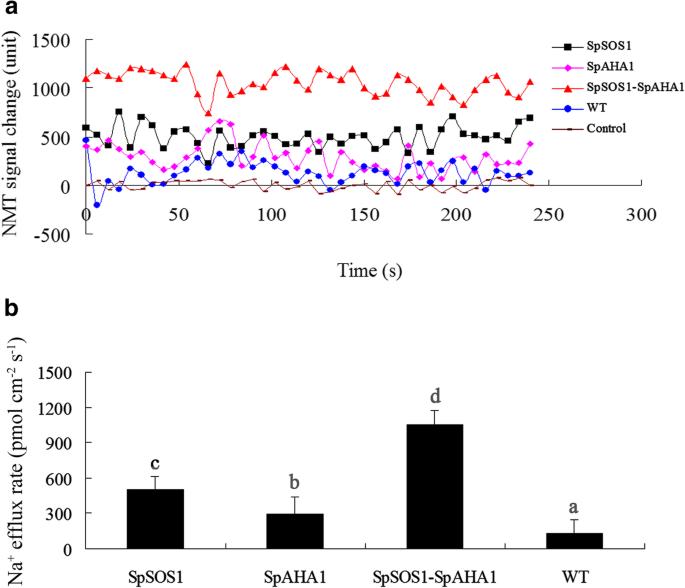
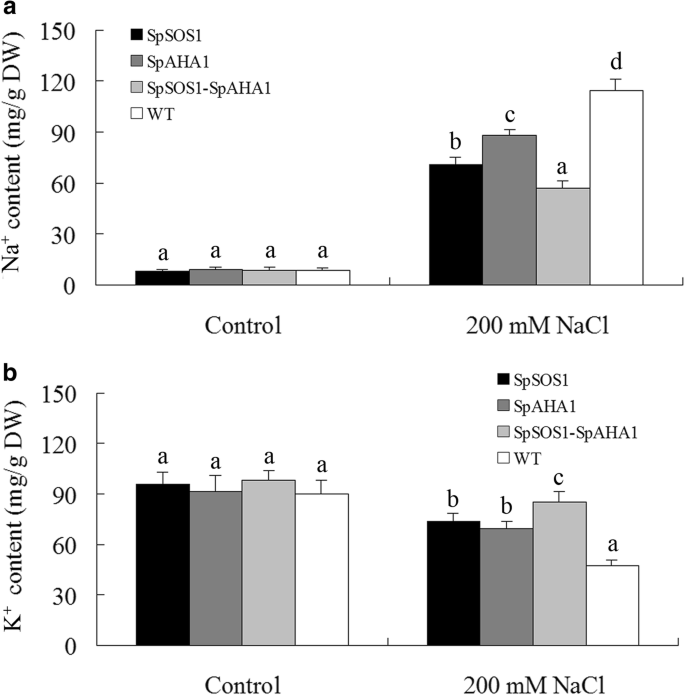
SOS1 mediates Na+excretion from cells and is a key halotolerance factor. SpSOS1 has been shown to be a PM-localized Na+/H+antiporter and capable of improving the growth of transgenic yeast cells under salt stress by decreasing the cellular Na+content [3].In this scenario, the roots from all tested plants grown in medium without NaCl displayed Na+uptake characteristics, but no significant differences in Na+flux activities at roots of transgenic and untransformed plants under unstressed condition were observed (Additional file3: Figure S3). On the contrary, NaCl treatment stimulated Na+effluxes at all tested roots.SpSOS1-expressing roots had faster Na+efflux relative to WT plants in saline conditions (Fig.4a, b), suggesting the extra Na+extrusion may be mediated by SpSOS1, which would result in the observed lower Na+content in theSpSOS1-transgenic plants than WT plants under salt stress (Fig.5a). SOS1-mediated Na+/H+exchange is powered by a proton gradient generated by an H+-ATPase. Therefore, a proton gradient generated by SpAHA1 (Fig.3) might catalyze native SOS1 (AtSOS1) to transport more Na+out of cells, which may be one reason for the higher Na+efflux rate in roots transformed withSpAHA1compared to WT plants in saline conditions (Fig.4). Roots co-expressingSpAHA1andSpSOS1had the highest Na+射流速度在所有的转基因植物tested, where the Na+efflux rate inSpSOS1-SpAHA1co-transgenic roots was 53 and 72% greater than the plants expressingSpSOS1orSpAHA1singly, respectively (Fig.4b). Correspondingly, the Na+levels in the transgenic plants were lower than in WT plants (Fig.5a). Therefore, it is reasonable thatSpSOS1-SpAHA1co-expression quickened Na+extrusion in the roots of and decreased Na+accumulation in transgenic plants compared toSpSOS1-orSpAHA1-expressing plants under saline conditions (Fig.4,5a). These results indicate SpAHA1 produced an additional proton gradient and, thus, promoted SpSOS1-mediated Na+extrusion inArabidopsisplants co-expressing both theSpAHA1andSpSOS1genes.
Na+flux in roots of NaCl-treatedArabidopsisplants. Seedlings were grown for 3 days on MS plates containing 100 mM NaCl. Na+flux in the roots was then measured using the NMT technique described in the Methods section. (a) Changes in the NMT signals are expressed as arbitrary units. (b) Na+flux is expressed as the amount of efflux per second per square centimeter (pmol•cm− 2•s− 1). Data are presented as mean ± SE of six replicates. Different letters above the columns indicate statistically significant differences at ap < 0.05 level among the different experimental cohorts. SpSOS1,SpSOS1-overexpressing plants; SpAHA1,SpAHA1-overexpressing plants; SpSOS1-SpAHA1,SpSOS1andSpAHA1co-expressing plants; WT, wild-type plants; Control, mock controls
Na+and K+content inArabidopsisplants. WT and transgenic plants were grown for 10 days in soil containing 0 or 200 mM NaCl. Na+(a) and K+(b) in the leaves of these plants were measured as described in the Methods section. Data are presented as mean ± SE of three replicates. Different letters above the columns indicate statistically significant differences at ap < 0.05 level among the different experimental cohorts. SpSOS1,SpSOS1-overexpressing plants; SpAHA1,SpAHA1-overexpressing plants; SpSOS1-SpAHA1,SpSOS1andSpAHA1co-expressing plants; WT, wild-type plants
SpSOS1-SpAHA1co-transgenic plants had higher K+retention under saline conditions
Among the physiological and biochemical processes in plant cells influenced by high salinity, nutrient imbalance is among the most deleterious resulting effects [41].The chemical and physical characteristics of sodium most resemble potassium among the nutrient elements. Therefore, excess Na+inhibits plant growth by interfering with cytosolic K+homeostasis. No differences in K+content was found among all the tested plants under normal conditions. However, upon salinity stress, the transgenic plants displayed less of a decrease in K+content than the WT plants. Furthermore, co-expression ofSpSOS1andSpAHA1most efficiently alleviated K+loss among the transgenic plants exposed to NaCl, where the K+content was highest in the leaves of plants co-expressingSpSOS1andSpAHA1(85 mg/g dry weight), followed by those fromSpSOS1-transgenic plants (73 mg/g dry weight), and thenSpAHA1-expressing plants (69 mg/g dry weight). WT plants had the lowest K+content (47 mg/g dry weight; Fig.5b).
SpSOS1-SpAHA1co-expression decreased malondialdehyde accumulation in transgenic plants
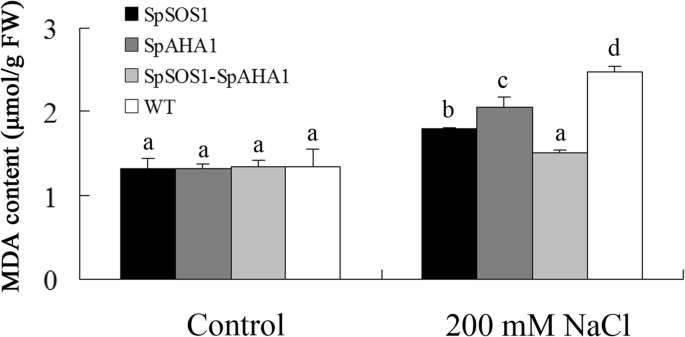
Salinity creates oxidative stress and excess reactive oxygen species can interfere with metabolism in the cytoplasm, such as by damaging membrane structures and destroying membrane integrity through lipid peroxidation [42].An indicator of membrane lipid oxidation, malondialdehyde (MDA) represents membrane lipid damage to some extent. Upon exposure to NaCl, the amount of MDA in the leaves of all tested plants increased, but MDA accumulation in theSpSOS1-SpAHA1co-expressing leaves was the lowest (Fig.6) at only 84, 74, and 61% of that inSpSOS1-expressing,SpAHA1-expressing, and WT plants under saline conditions. These results indicateSpSOS1andSpAHA1coordination could more efficiently reduce oxidative damage to membranes from salinity stress in transgenic plants.
Malondialdehyde content in leaves. Two-week-old WT and transgenicArabidopsisseedlings were treated with 200 mM NaCl for 7 days and then their leaves were harvested. Malondialdehyde content in the leaves was measured as described in the Methods section. Data are presented as mean ± SE of three replicates. Different letters above the columns indicate statistically significant differences at ap < 0.05 level among the different experimental cohorts. SpSOS1,SpSOS1-overexpressing plants; SpAHA1,SpAHA1-overexpressing plants; SpSOS1-SpAHA1,SpSOS1andSpAHA1co-expressing plants; WT, wild-type plants
Discussion
Plants grown under K+deficiency substitute it by Na+, especially some halophyte species can use Na+for stomata operation instead of K+[43].However, when there is excess sodium in the cytosol, it interferes with some key metabolic processes and eventually inhibits the growth and development of the plant. To tolerate high Na+levels, plant cells must be capable of removing Na+from the cytoplasm through some physiological processes. In one of these processes, cytoplasmic Na+can be imported into vacuoles through tonoplast Na+/H+antiporter NHXs using an electrochemical gradient established by a vacuolar H+-ATPase and H+-PPase (AVP/VP) using protons. The sequestration of Na+into vacuoles not only prevents the deleterious effects resulting from Na+in the cytoplasm, but also lets the plants use Na+as an osmoticum, which helps maintain the osmotic potential that drives water into the cells [39,44].Therefore, tonoplast NHX antiporters and H+-pumps have important functions in plant responses to salt stress. In transgenic plants, overexpression of genes encoding vacuolar Na+/H+antiporters or H+-PPases enhances salt tolerance [39,42].Furthermore, co-expression of AVP and NHX may better improve the growth of transgenic plants exposed to salt stress through more efficient compartmentalization of Na+into vacuoles than when NHX or AVP are expressed alone. Co-expression ofZxNHXandZxVP1–1confers better salt tolerance to transformed sugar beet and lotus plants [42,45].NHX1-AVP1co-transgenic rice grows better under salt stress than rice plants expressing only one of these genes [46,47].Tobacco plants co-expressingTNHXS1andTVP1have higher salt tolerance than transgenic plants expressingTNHXS1orTVP1alone [48].Another mechanism contributing to Na+extrusion is the PM Na+/H+antiporter SOS1. The Na+extrusion mediated by SOS1 is also driven by electrochemical gradients of protons generated by a PM H+-pump (H+-ATPase, AHA). The overexpression ofSOS1significantly improves the salt tolerance of transgenic grapevine compared to WT plants [49].Overexpression of theSOS1gene in tobacco plants increases salt tolerance by maintaining a lower Na+content [50] and the growth ofArabidopsisplants overexpressingSOS1is better than that of WT plants under salt stress [51].It has been reported that overexpression ofPeHA1(H+-ATPase 1),a poplar gene encoding a PM-localized H+-ATPase, enhances the salt tolerance of transgenicArabidopsis[52].These studies suggest that co-expression of bothSOS1andAHAin transgenic plants should more effectively increase salinity tolerance just as co-expression of vacuolar NHX and AVP results in higher salt tolerance. In the present investigation,SpAHA1-transgenic roots had faster H+efflux than WT plants under salt stress (Fig.3), suggesting SpAHA1 enhanced proton efflux and generated an additional proton gradient that acted as a driving force for Na+/H+exchange mediated by SpSOS1. More interestingly, the magnitude of net H+flux is in 3 to 4 pmol•cm− 2•s− 1range (Fig.3), while Na+flux is around 1000 pmol•cm− 2•s− 1; the stoichiometric ratio for Na+/H+交换的年代OS1 protein is 1H+:1Na+, so such tiny increase in H+flux (from 2 to 4 pmol•cm− 2•s− 1) may not cause such a massive flux of Na+. Net H+fluxes at roots was determined in the present study, that is, the data for net H+efflux is equal to total H+efflux minus total H+influx at the roots, suggesting that H+-ATPase mediated H+efflux is likely balanced by H+uptake through SOS1 transporters. These suggest that SpAHA1 might provided more H+gradient than the shown data of net H+efflux in transgenic plant exposed to salinity stress, resulting in faster Na+efflux from cells, consequently Na+content in transgenic plants was lower than that in wild type plants (Fig.5a). Overexpression ofSpSOS1in transgenicArabidopsisaccelerated Na+efflux in the roots (Fig.4a, b), resulting in decreased Na+content in the transgenic plants compared to the WT plants (Fig.5a)。有趣的是,H+efflux in theSpSOS1-expressing roots was faster than in the WT orSpAHA1-transgenic roots (Fig.3), suggesting that increased Na+extrusion mediated by SpSOS1 might regulate H+-pumping activity via feedback at the PM. This is because the Na+/H+exchange mediated by SOS1 is dependent on energy and driven using a PM-localized H+-ATPase-driven proton motive force [53].The H+and Na+efflux rates in the roots ofArabidopsisplants co-expressingSpAHA1andSpSOS1were highest among all the transgenic plants (Figs.3and4), leading to the lowest Na+content in the co-transformed plants relative to other transgenic plants expressing onlySpSOS1orSpAHA1(Fig.5a). In response to NaCl treatment, the biomass of the transgenicArabidopsisplants co-expressingSpSOS1andSpAHA1was greater than the biomasses of the single-gene expressing plants (Fig.2). Taken together, the higher rate of Na+extrusion, lower Na+levels, and better growth of theSpAHA1-SpSOS1co-expressing plants compared to the singleSpAHA1orSpSOS1gene transgenic plants provides direct genetic evidence that SOS1 and AHA function in a cooperative manner to inhibit Na+accumulation in the cytosol and play important roles in plant adaption to highly saline conditions.
High soil salinity is characterized by high soluble salt concentrations, of which sodium salt is the most soluble and widespread salt [44].Excessive sodium ions in soils can enter into plant cells and then interference with some critical biochemical and physiological processes. The most deleterious effect of salinity is ion toxicity [41].K+is a necessary macronutrient that has a critical role in the growth and development of plants [54].Due physicochemical similarities between Na+and K+, Na+can compete with K+for binding sites important in critical cytoplasmic physiological and biochemical processes [55].In particular, Na+inhibits the activity of many K+-dependent enzymes [56] and, therefore, excess Na+can inhibit K+-associated activities in the cytosol [55].It is hypothesized that plant survival in the presence of salt stress requires a high K+/Na+ratio in the cytoplasm [57].Therefore, limiting Na+influx into cells may facilitate plant growth under salt stress [58,59].Under high salt conditions, the PM potential becomes depolarized, which encourages passive Na+influx into cells and K+efflux out of cells. H+-ATPase-generated electrochemical potential gradients across PMs can repolarize PMs following NaCl-induced depolarization [39].Therefore, maintenance of the PM potential using H+-ATPases can reduce the Na+influx via depolarization-activated non-selective cation channels (NSCCs) and K+efflux via K+outward rectifiers (KORs) and NSCCs [60].The net H+efflux from root cells inSpAHA1-transgenic plants occurred at a higher rate relative to WT plant roots, suggesting SpAHA1 increased the H+-ATPase activity and electrochemical potential and, thus, the H+gradient across the PM. This may reduce Na+influx and K+efflux and, correspondingly, theSpAHA1-transgenic plants had less Na+and more K+than WT plants (Fig.5a, b). In addition to Na+extrusion, SOS1 is also involved in K+homeostasis in plant cells under high salt conditions. Transgenic tobacco plants expressingSbSOS1contain less Na+, but more K+, in their roots than WT plants under high salt stress [50].Horie et al. [61] suggested SOS1 plays a primary role facilitating high-affinity absorption of K+into roots. SOS1 is necessary for protecting K+uptake and is involved in K+homeostasis maintenance in cells under salinity stress [19,62].Overexpression ofTaSOS1confers salt tolerance to transgenic tobacco plants by decreasing the Na+and increasing the K+levels [31].Arabidopsisroots expressingSpSOS1displayed faster Na+efflux than WT plant roots under saline condition (Fig.4), suggesting SpSOS1 was responsible for the extra Na+extrusion. The faster H+efflux in the roots of plants expressingSpSOS1may aid repolarization following NaCl-induced depolarization of the PM, thus decreasing Na+influx and K+efflux [60].These actions may have ledSpSOS1-transgenic plants to contain less Na+and more K+relative to WT plants under salt treatment. Therefore, faster H+and Na+efflux in the roots also resulted in retention of more K+and a lower Na+concentration in cytosol ofSpSOS1-SpAHA1co-transgenicArabidopsisplants compared to plants expressingSpSOS1orSpAHA1alone (Figs.3and4). This led toArabidopsisplants co-expressingSpSOS1andSpAHA1to have higher a K+/Na+level than the transgenic plants with onlySpSOS1orSpAHA1, which is strong evidence of salt tolerance. These results suggest SOS1 and AHA1 facilitate more efficient prevention of K+loss and enhance Na+extrusion and thereby contribute to better salt tolerance.
Another deleterious effect of salinity stress in plants is associated with oxidative stress [39].Accumulation of ROS (reactive oxygen species) is toxic in cells. Therefore, intracellular ROS levels are tightly regulated under normal conditions through a number of intracellular peroxidative and antioxidative reactions within the cell. Salinity can disrupt the ROS production and scavenging balance, resulting in ROS accumulation, which can negatively affect cellular structures and metabolism [63,64].In order to protect cells from salinity-induced oxidative damage, excess ROS is scavenged by antioxidant molecules and enzymes. RCD1 (Radical-induced cell death) is a regulator of responses to oxidative stress and protects cells from oxidative damage caused by H2O2, diamide, and tert-butyl peroxide [65,66,67].SOS1 functions in tolerance to oxidative stress by interacting with RCD1 and regulating expression of certain genes associated with oxidative-stress tolerance inArabidopsis[66].血红素氧合酶(HO)是一个重要的因素lant antioxidant defense systems. Overexpression of theAtHOgene enhancesArabidopsistolerance to salt by increasing PM H+-ATPase activity and expression [68].过量的活性氧会破坏膜结构由牛idizing lipids in the PM, leading some key metabolites abnormally leak out of cells. ROS could disturb ion homeostasis in cells by inducing the efflux of several cations [69,70,71].Coskun et al. [53] found NaCl-induced efflux of K+was a result of a lack of PM integrity in rice. This indicates the maintenance of PM stability has a key role in plant salt tolerance. In the present investigation, both SpSOS1 and SpAHA1 prevented the accumulation of MDA in transgenic plants following NaCl treatment, but plants co-expressingSpSOS1andSpAHA1had a more drastic decrease in MDA content under salt stress than plants expressing only one of these genes. This suggests SpSOS1 and SpAHA1 coordinate in transgenicArabidopsisand ameliorate salt toxicity by more efficiently alleviating oxidative damage to the PM generated by salinity stress.
Conclusions
Arabidopsisplants co-expressingSpSOS1andSpAHA1had higher K+and lower MDA levels than plants transformed with onlySpSOS1orSpAHA1and, thus, grew better under salt stress. The coordinated action of these genes might be a novel and effective method for increasing the salt tolerance of crops.
Methods
Plasmid construction
TheSpAHA1andSpSOS1genes were separately cloned fromS. portulacastrumand inserted into plasmids p414 (p414-SpAHA1) and p416 (p416-SpSOS1) in our recent investigation [3].Plant vectors expressingSpAHA1orSpSOS1alone or together bicistronicly were constructed as described in Additional file4: Figure S4. (1) Amplification of theSpSOS1gene was performed using the p416-SpSOS1plasmid as the template and the primers SpSOS1-F and SpSOS1-R (Additional file5: Table S1). The amplified gene was inserted into the pCAMBIA1300 vector betweenSalI andKpnI restriction sites, generating pCAMBIA1300-SpSOS1. (2) A fragment containing a constitutive promoter (cauliflower mosaic virus 35S promoter), theSpSOS1gene, and the NOS terminator was excised from the pCAMBIA1300-SpSOS1plasmid usingPstI andEcoR I restriction enzymes and transferred into the plant expression vector pCAMBIA1304 between the same restriction sites. The resulting plasmid was named pCAMBIA1304-SpSOS1. (3) TheSpAHA1gene was amplified using the p414-SpAHA1plasmid as the template and the primers SpAHA1-F and SpAHA1-R (Additional file5: Table S1) and inserted into the pCAMBIA1304 and pCAMBIA1304-SpSOS1plasmids between theSpeI andEco72 I restriction sites to replace theGUS(β-glucuronidase) gene. The resulting plasmids were designated pCAMBIA1304-SpAHA1and pCAMBIA1304-SpSOS1-SpAHA1, respectively. The plasmids were all verified by sequencing.
Arabidopsistransformation and identification
The three recombinant plasmids described above were added to a 100 mM CaCl2solution containing competentAgrobacterium tumefaciensGV3101 cells. The plasmids were then introduced into theAgrobacteriumcells via heat shock (42 °C). Finally, the above three expression cassettes were transformed intoArabidopsis thaliana(Col-0) by infecting flower buds with theAgrobacteriumcells containing the recombinant plasmids [72].T0-generation seeds were screened initially on MS (Murashige & Skoog) medium supplemented with 50 μg/mL hygromycin B. DNA was purified from the candidate lines and used as template for PCR (polymerase chain reaction) amplification with specific primer pairs to identify the different transgenic plants. The primers are listed in Additional file5: Table S1. All the transgenic lines were furthermore verified by PCR using primers specific for thehygBmarker gene, HygB-TF and HygB-TR (Additional file5: Table S1). Total RNA was purified from the transgenic lines andSpAHA1andSpSOS1expression was assessed by RT-PCR (reverse transcription PCR) with a housekeeping gene,Actin,as an internal control. The primers for theSpAHA1(SpAHA1-RT-F and SpAHA1-RT-R),SpSOS1(SpSOS1-RT-F and SpSOS1-RT-R), andActin(Actin-RT-F and Actin-RT-F) genes are listed in Additional file5: Table S1.PCR条件如下:94°C 2米n, followed by 28 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and a final extension at 72 °C for 5 min. The resulting PCR products were assessed by agarose gel electrophoresis.
Cultivation and salt treatment of transgenic and WT plants
To analyze the salt tolerance of transgenic and WT plants, seeds from T3 homozygous transgenic lines (expressing singleSpSOS1, singleSpAHA1,and bothSpSOS1andSpAHA1) and untransformed plants were germinated on MS plates in a growth chamber (22 °C with a 16 h light / 8 h dark cycle and a light intensity of 100 μmol•m− 2•s− 1). After 5 days, the seedlings grown on MS plates were transferred onto MS plates containing 0, 50, 75, and 100 mM NaCl and allowed to grow for 2 weeks. Then the root length, number of lateral roots, and fresh seedling weights were measured. In addition, 10-day-old seedlings were transferred to a mixture of organic soil and sand (3:1,v/v) in pots (4 seedlings/pot) and grown in a greenhouse with long-day conditions (16 h light/8 h dark at 22 °C and a light strength of 150 μmol•m− 2•s− 1) for 4 weeks. The pots containing the plants were then put into water containing 0 or 200 mM NaCl. Ten days post-NaCl treatment, the treated plants were photographed and their fresh weights were determined.
Determination of Na+and K+content inArabidopsisplants
At the end of the NaCl treatment, the WT and transgenic plants were separately collected. The Na+and K+in the samples were measured using atomic absorption spectrometry as described in a previous work [31].
Measurement of Na+and H+flux in roots
Seven-day-old uniform T3 seedlings, which had been grown on MS plates, were transferred to MS medium containing 100 mM NaCl and grown for 3 days, and then the roots of salt stressed seedlings were put into measurement buffer to balance for 10 min, after that net H+and Na+fluxes were measured in the YoungerUSA Xuyue (Beijing) BioFunction Institute by using Non-invasive Micro-test Technology (NMT100 Series, Xuyue (Beijing) Sci. & Tech. Co., Ltd., Beijing, China) Software.
H+, Na+-selective microsensors were prepared as described previously [60].Pre-pulled and silanized microsensor (Φ4.5 ± 0.5 μm, XY-CGQ-01, YoungerUSA) were first filled with a backfilling solution (H+: 15 mM NaCl + 40 mM KH2PO4, pH 7.0; Na+: 250 mM NaCl) to a length of approximately 1.0 cm from the tip. Then the microsensors were front filled with 40–50 μm columns of selective liquid ion-exchanger (LIX) (H+: XY-SJ-H; Na+: XY-SJ-Na; all from YoungerUSA). An Ag/AgCl wire microsensor holder YG003-Y11 (YoungerUSA) was inserted in the back of the microsensor to make electrical contact with the electrolyte solution. YG003-Y11 (YoungerUSA) was used as the reference microsensor. Prior to the flux measurement, the microsensor were calibrated with cultural media having different concentrations of H+(pH 6.0 and pH 7.0) or Na+(5 mM and 0.5 mM), respectively. Only microsensor with a Nernstian slope >50 mV/decade were used in our study. The same microsensors were calibrated again according to the same procedure and standards after each test. After that, net ion fluxes were recorded on the root meristematic zones, 120 μm from the tip where SOS1 activity was the highest [73], in 5 mL measurement buffer (0.1 mM KCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM NaCl, 0.3 mM MES (2-(N-Morpholino) ethanesulfonic acid) and 0.2 mM Na2SO4, pH 6.5). Net H+and Na+flux was calculated by Fick’s law of diffusion [60].Six biological repeats were performed for each analysis.
Assays of malondialdehyde content
Two-week-old T3 transgenic and untransformedArabidopsisseedlings were grown in the presence of 200 mM NaCl for 7 days. Malondialdehyde (MDA) content in the leaves was measured using the thiobarbituric acid method previously described by Dhindsa and Matowe [74].
Statistical analysis
Two-tailed Student’s t-tests were used to analyze the data. The results are expressed as mean ± SE and differences with aP-value < 0.05 were considered statistically significant. At least three biological replicates were performed for each experiment.
Abbreviations
- AHA:
-
H+-ATPase
- AVP/VP:
-
Vacuolar H+-PPase
- GUS:
-
β-glucuronidase
- HA:
-
H+-ATPase
- HO:
-
Haem oxygenase
- KOR:
-
K+outward rectifier
- MDA:
-
Malondialdehyde
- MES:
-
2-(N-Morpholino) ethanesulfonic acid
- MS:
-
Murashige & Skoog
- NHX:
-
Na+/H+antiporter
- NSCC:
-
Non-selective cation channels
- PCR:
-
Polymerase chain reaction
- PM:
-
Plasma membrane
- RCD:
-
Radical-induced cell death
- RNAi:
-
RNA interference
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SOS:
-
Salt overly sensitive
- WT:
-
Wild-type
References
- 1.
Kronzucker HJ, Coskun D, Schulze LM, Wong JR, Britto DT. Sodium as nutrient and toxicant. Plant Soil. 2013;369:1–23.
- 2.
Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytol. 2008;179:945–63.
- 3.
Zhou Y, Yin X, Duan R, Hao G, Guo J, Jiang X. SpAHA1 and SpSOS1 coordinate in transgenic yeast to improve salt tolerance. PLoS One. 2015;10:e0137447.
- 4.
Fuglsang AT, Paez-Valencia J, Gaxiola RA. Plant proton pumps: regulatory circuits involving H+-ATPase and H+-PPase. In: Geisler M, Venema K, editors. Transporters and pumps in plant signaling. Berlin: Springer; 2010. p. 39–64.
- 5.
Gaxiola RA, Palmgren MG, Schumacher K. Plant proton pumps. FEBS Lett. 2007;581:2204–14.
- 6.
Arango M, Gevaudant F, Oufattole M, Boutry M. The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta. 2003;216:355–65.
- 7.
Axelsen KB, Palmgren MG. Inventory of the superfamily of P-type ion pumps inArabidopsis. Plant Physiol. 2001;126:696–706.
- 8.
Bose J, Rodrigo-Moreno A, Lai D, Xie Y, Shen W, Shabala S. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species,Atriplex lentiformisandChenopodium quinoa. Ann Bot. 2015;115:481–94.
- 9.
Ding M, Hou P, Shen X, Wang M, Deng S, Sun J, Xiao F, Wang R, Zhou X, Lu C, et al. Salt-induced expression of genes related to Na+/K+and ROS homeostasis in leaves of salt-resistant and salt sensitive poplar species. Plant Mol Biol. 2010;73:251–69.
- 10.
Niu X, Narasimhan M, Salzman R. NaCl regulation of plasma membrane H+-ATPase gene expression in glycophyte and halophyte. Plant Physiol. 1993;103:712–8.
- 11.
Sahu B, Shaw B. Salt-inducible isoform of plasma membrane H+-ATPase gene in rice remains constitutively expressed in natural halophyte,Suaeda maritime. J Plant Physiol. 2009;166:1077–89.
- 12.
Chen M, Song J, Wang B. NaCl increases the activity of the plasma membrane H+-ATPase in C3 halophyteSuaeda salsacallus. Acta Physiol Plant. 2010;36:27–36.
- 13.
Lopez-Pérez L, Martinez-Ballesta M, Maurel C, Carvajal M. Changes in plasma membrane lipids, aquaporins and proton pump of broccoli roots, as an adaptation mechanism to salinity. Phytochemistry. 2009;70:492–500.
- 14.
Palmgren MG. Plant plasma membrane H1-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:817–45.
- 15.
Pons R, Cornejo MJ, Sanz A. Differential salinity-induced variations in the activity of H+-pumps and Na+/H+antiporters that are involved in cytoplasm ion homeostasis as a function of genotype and tolerance level in rice cell lines. Plant Physiol Biochem. 2011;49:1399–409.
- 16.
Vitart V, Baxter I, Doerner P, Harper JF. Evidence for a role in growth and salt resistance of a plasma membrane H+-ATPase in the root endodermis. Plant J. 2001;27:191–201.
- 17.
Gevaudant F, Duby G, Stedingk E, Zhao R, Morsomme P, Boutry M. Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance. Plant Physiol. 2007;144:1763–76.
- 18.
Wu SJ, Ding L, Zhu JK. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell. 1996;8:617–27.
- 19.
Xu H, Jiang X, Zhan K, Cheng X, Chen X, Pardo JM, Cui D. Functional characterization of a wheat plasma membrane Na/H antiporter in yeast. Arch Biochem Biophys. 2008;473:8–15.
- 20.
Oh DH, Leidi E, Zhang Q, Hwang SM, Li Y, Quintero FJ, Jiang X, D’Urzo MP, Lee SY, Zhao Y, et al. Loss of halophytism by interference with SOS1 expression. Plant Physiol. 2009;151:210–22.
- 21.
Olias R, Eljakaoui Z, Li J, Morales PA, Marin-Manzano MC, Pardo JM, Blelver A. The plasma membrane Na+/H+antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+between plant organs. Plant Cell Environ. 2009;32:904–16.
- 22.
Tang RJ, Liu H, Bao Y, Lv QD, Yang L, Zhang H. The woody plant poplar has a functionally conserved salt overly sensitive pathway in response to salinity stress. Plant Mol Biol. 2010;74:367–80.
- 23.
Fraile-Escanciano A, Kamisugi Y, Cuming AC, Rodríguez-Navarro A, Benito B. The SOS1 transporter ofPhyscomitrella patensmediates sodium efflux in planta. New Phytol. 2010;188:750–61.
- 24.
Feki K, Quintero FJ, Pardo JM, Masmoudi K. Regulation of durum wheat Na+/H+exchanger TdSOS1 by phosphorylation. Plant Mol Biol. 2001;76:545–56.
- 25.
Li Q, Tang Z, Hu Y, Yu L, Liu Z, Xu G. Functional analyses of a putative plasma membrane Na+/H+antiporter gene isolated from salt tolerantHelianthus tuberosus. Mol Biol Rep. 2014;41:5097–108.
- 26.
Quintero FJ, Martinez-Atienza J, Villalta I, Jiang X, Kim MY, Ali Z, Fujii H, Mendoza I, Yun DJ, Zhu JK, et al. Activation of the plasma membrane Na/H antiporter salt-overly-sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc Natl Acad Sci U S A. 2011;108:2611–6.
- 27.
Martinéz-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, Quintero FJ. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007;143:1001–12.
- 28.
Shi H, Ishitani M, Kim C, Zhu JK. TheArabidopsis thalianasalt tolerance geneSOS1encodes a putative Na+/H+antiporter. Proc Natl Acad Sci U S A. 2000;97:6896–901.
- 29.
Zhu JK, Liu J, Xiong L. Genetic analysis of salt tolerance inArabidopsis: evidence for a critical role of potassium nutrition. Plant Cell. 1998;10:1181–91.
- 30.
Shi H, Lee BH, Wu SJ, Zhu J. Overexpression of a plasma membrane Na+/H+antiporter gene improves salt tolerance inArabidopsis thaliana. Nat Biotechnol. 2003;21:81–5.
- 31.
Zhou Y, Lai Z, Yin X, Yu S, Xu Y, Wang X, Cong X, Luo Y, Xu H, Jiang X. Hyperactive mutant of a wheat plasma membrane Na+/H+antiporter improves the growth and salt tolerance of transgenic tobacco. Plant Sci. 2016;253:176–86.
- 32.
易XP,太阳Y,杨问,郭美联社,常将王Dng Z, Jin X, Wang LM, Yu JL, et al. Quantitative proteomics ofSesuvium portulacastrumleaves revealed that ion transportation by V-ATPase and sugar accumulation in chloroplast played crucial roles in halophyte salt tolerance. J Proteome. 2014;99:84–100.
- 33.
Slama I, Ghnaya T, Savoure A, Abdelly C. Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation ofSesuvium portulacastrum. C R Biol. 2008;331:442–51.
- 34.
Rabhi M, Ferchichi年代,Jouini J, Hamrouni MH, KoyroHW, Ranieri A, Abdelly C, Smaoui A. Phytodesalination of a salt-affected soil with the halophyteSesuvium portulacastrumL. to arrange in advance the requirements for the successful growth of a glycophytic crop. Bioresour Technol. 2010;101:6822–8.
- 35.
Lokhande VH, Nikam TD, Suprasanna P. Biochemical, physiological and growth changes in response to salinity in callus cultures ofSesuvium portulacastrumL. Plant Cell Tiss Org. 2010;102:17–25.
- 36.
Nawaz I, Iqbl M, Hakvoort HWJ, Bliek M, de Boer B, Schat H. Expression levels and promoter activities of candidate salt tolerance genes in halophyte and glycophytic Brassicaceae. Environ Exp Bot. 2014;99:59–66.
- 37.
Fan Y, Wan S, Jiang Y, Xia Y, Chen X, Gao M, Cao Y, Luo Y, Zhou Y, Jiang X. Over-expression of a plasma membrane H+-ATPase SpAHA1 conferred salt tolerance to transgenicArabidopsis. Protoplasma. 2018;255:1827–37.
- 38.
Zhou Y, Yin X, Wan S, Hu Y, Hu Y, Xie Q, Li R, Zhu B, Fu S, Guo J, Jiang X. TheSesuvium portulacastrumplasma membrane Na+/H+antiporter SpSOS1 complemented the salt sensitivity of transgenicArabidopsis sos1mutant plants. Plant Mol Biol Rep. 2018;36:553–63.
- 39.
Mansour MMF. The plasma membrane transport systems and adaptation to salinity. J Plant Physiol. 2014;171:1787–800.
- 40.
Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, Kristiansen KA, Bych K, Schulz A, Shabala S, Schumaker KS, et al.Arabidopsisprotein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell. 2007;19:1617–34.
- 41.
Munns R, Tester M. Mechanism of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–81.
- 42.
Wu GQ, Feng RJ, Wang SM, Wang CM, Bao AK, Wei L, Yuan HJ. Co-expression of xerophyteZygophyllum xanthoxylumZxNHX and ZxVP1–1 confers enhanced salinity tolerance in chimeric sugar beet (Beta vulgarisL.). Front Plant Sci. 2015;6:581.
- 43.
Hedrich R, Shabala S. Stomata in a saline world. Curr Opin Plant Biol. 2018;46:87–95.
- 44.
Almeida DM, Oliveira MM, Saibo NJM. Regulation of Na+and K+homeostasis in plants: towards improved salt stress tolerance in crop plants. Genet Mol Biol. 2017;40:326–45.
- 45.
Bao AK, Wang YW, Xi JJ, Liu C, Zhang JI, Wang SM. Co-expression of xerophyteZygophyllum xanthoxylumZxNHX and ZxVP1-1 enhances salt and drought tolerance in transgenicLotus corniculatusby increasing cations accumulation. Funct Plant Biol. 2014;41:203–14.
- 46.
Zhao FY, Zhang XJ, Li PH, Zhao YX, Zhang H. Co-expression of theSuaeda salsa SsNHX1andArabidopsis AVP1带来更大的盐耐受性transgenic rice than the singleSsNHX1. Mol Breeding. 2006;17:341–53.
- 47.
Liu SP, Zheng LQ, Xue YH, Zhang Q, Wang L, Shou HX. Overexpression ofOsVP1andOsNHX1increases tolerance to drought and salinity in rice. J Plant Biol. 2010;53:444–52.
- 48.
Gouiaa S, Khoudi H, Leidi EO, Pardo JM, Masmoudi K. Expression of wheat Na+/H+antiporterTNHXS1and H+-pyrophosphataseTVP1genes in tobacco from a bicistronic transcriptional unit improves salt tolerance. Plant Mol Biol. 2012;79:137–55.
- 49.
Upadhyay A, Upadhyay AK, Bhirangi R. Expression of Na+/H+antiporter gene in response to water and salinity stress in grapevine rootstocks. Biol Plant. 2012;56:762–6.
- 50.
Yadav NS, Shukla P, Jha A, Agarwal P, Jha B. TheSbSOS1gene from the extreme halophyteSalicornia brachiataenhances Na+loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biol. 2012;12:188.
- 51.
Yang Q, Chen Z, Zhou X, Yin H, Xin X, Hong X, Zhu JK, Gong ZZ. Overexpression ofSOS(salt overly sensitive) genes increases salt tolerance in transgenicArabidopsis. Mol Plant. 2009;2:22–31.
- 52.
Wang M, Wang Y, Sun J, Ding M, Deng S, Hou P, Ma X, Zhang Y, Wang F, Sa G, et al. Overexpression ofPeHA1enhances hydrogen peroxide signaling in salt-stressedArabidopsis. Plant Physiol Biochem. 2013;71:37–48.
- 53.
Coskun D, Britto D, Jean Y, Kabir I, Tolay I, Torun AA, Kronzucker HJ. K+efflux and retention in response to NaCl stress do not predict salt tolerance in contrasting genotypes ofrice (Oryza sativaL.). PLoS One. 2013;8:e57767.
- 54.
Barragan V, Leidi EO, Andrés Z, Rubio L, De Luca A, Fernandez JA, Cubero B, Pardo JM. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function inArabidopsis. Plant Cell. 2012;24:1127–42.
- 55.
Marschner H. Mineral nutrition of higher plants. Ann Bot. 1995;78:527–8.
- 56.
Duggleby RG, Dennis DT. Pyruvate kinase, a possible regulatory enzyme in higher plants. Plant Physiol. 1973;52:312–7.
- 57.
Maathuis FJM, Amtmann A. K+nutrition and Na+toxicity: the basis of cellular K+/Na+ratios. Ann Bot. 1999;84:123–33.
- 58.
Lutts S, Kinet J, Bouharmont J. NaCl-induced senescence in leaves of rice (Oryza sativaL.) cultivars differing in salinity resistance. Ann Bot. 1996;78:389–98.
- 59.
Tester M, Davenport RJ. Na+tolerance and Na+transport in higher plants. Ann Bot. 2003;91:503–27.
- 60.
Sun J, Dai S, Wang R, Chen S, Li N, Zhou X, Lu C, Shen X, Zheng X, Hu Z, et al. Calcium mediates root K+/Na+homeostasis in poplar species differing in salt tolerance. Tree Physiol. 2009;29:1175–86.
- 61.
Horie T, Karahara I, Katsuhara M. Salinity tolerance mechanisms in glycophytes: an overview with the central focus on rice plants. Rice. 2012;5:11.
- 62.
Qi Z, Spalding EP. Protection of plasma membrane K+transport by the salt overly sensitive1 Na+/H+antiporter during salinity stress. Plant Physiol. 2004;136:2548–55.
- 63.
Chaves MM, Oliveira MM. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot. 2004;55:2365–84.
- 64.
Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103:551–60.
- 65.
Belles-Boix E, Babiychuk E, Van Montagu M, Inze D, Kushnir S. CEO1 a new protein fromArabidopsis thaliana, protects yeast against oxidative damage. FEBS Lett. 2000;482:19–24.
- 66.
Katiyar-Agarwal S, Zhu J, Kim K, Agarwal M, Fu X, Huang A, Zhu JK. The plasma membrane Na+/H+antiporter SOS1 interacts with RCD1 and functions in oxidative stress tolerance inArabidopsis. Proc Natl Acad Sci U S A. 2006;103:18816–21.
- 67.
Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann HJ, Kangasjarvi J. Ozone-sensitiveArabidopsis rcd1mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell. 2000;12:1849–62.
- 68.
Bose J, Xie Y, Shen W, Shabala S. Haem oxygenase modifies salinity tolerance inArabidopsisby controlling K+retention via regulation of the plasma membrane H+-ATPase and by altering SOS1 transcript levels in roots. J Exp Bot. 2013;64:471–81.
- 69.
Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V.Arabidopsisroot K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci. 2010;123:1468–79.
- 70.
Demidchik V, Maathuis FJM. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol. 2007;175:387–404.
- 71.
Velarde-Buendía AM, Shabala S, Cvikrova M, Dobrovinskaya O. Salt-sensitive and salt-tolerant barley varieties differ in the extent of potentiation of the ROS-induced K+efflux by polyamines. Plant Physiol Biochem. 2012;61:18–23.
- 72.
Clough SJ, Bent AF. Floral dip: a simplified method forAgrobacterium- mediated transformation ofArabidopsis thaliana. Plant J. 1998;16:735–43.
- 73.
Shabala L, Cuin TA, Newman IA, Shabala S. Salinity-induced ion flux patterns from the excised roots ofArabidopsis sosmutants. Planta. 2005;222:1041–50.
- 74.
Dhindsa RS, Matowe W. Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot. 1981;32:79–91.
Acknowledgements
We thank Boston Professional Group (BPG) Editing for English language editing. We also thank the reviewers and editors for helpful comments on earlier drafts of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31660253 to XJ), the Scientific and Technological Foundation of Hainan Province (HNGDhs201502 to XJ), the Foundations of Hainan University and Shandong Normal University (hdkytg201706 to XJ and JS), and Startup funding from Hainan University (KYQD(ZR)1845 to YZ). The above funding was used for the design of the study and collection, analysis, and interpretation of data in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from corresponding authors on reasonable request.
Author information
Affiliations
Contributions
XJ, YZ and ZW conceived and designed the experiments. YF, XY, QX and YX performed the experiments. YZ and JS made substantial contributions to the data analysis. XJ and JS supervised, wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
出版商的注意
关于文书期刊上施普林格自然保持中立isdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1.Molecular identification of transgenic plants. DNA was purified from transgenic and WT plant leaves. (a) PCR identification ofSpSOS1transgenic plants. M: DL2000 marker (Sangon Biotech, China; No. B600335); 1–12, different transgenic lines (lines 1–12); 13, negative control (WT plants). (b) PCR identification ofSpAHA1transgenic plants. M: DL2000 marker; 1–11, different transgenic lines (lines 1–11); 12, negative control (WT plants). (c) PCR identification ofSpSOS1andSpAHA1co-expressing plants. M: DL2000 marker; 1–10, different transgenic lines (lines 1–10); 11, negative control (WT plants). PCR amplification was performed using primers specific forSpSOS1,SpAHA1,orhygB基因(预期大小的980、916和750个基点,分别地ectively) with the corresponding DNA serving as the template. The PCR products were assessed by agarose gel electrophoresis. (TIF 835 kb)
Additional file 2:
Figure S2.Expression ofSpSOS1andSpAHA1genes in transgenicArabidopsislines. Total RNA was purified from leaves from the T3 generation of transgenic plants and used for RT-PCR analysis. TheArabidopsis Actingene served as an internal control. (a) Expression of theSpSOS1gene inSpSOS1-transgenic plants was analyzed by RT-PCR. 1–12, different transgenic lines (lines 1–12). (b) Expression ofSpAHA1gene inSpAHA1-transgenic plants as analyzed by RT-PCR. 1–11, different transgenic lines (lines 1–11). (c) Expression ofSpSOS1andSpAHA1genes inSpSOS1-SpAHA1co-expressing plants as analyzed by RT-PCR. 1–10, different transgenic lines (lines 1–10). (TIF 531 kb)
Additional file 3:
Figure S3.Na+flux in roots ofArabidopsisplants grown in media without NaCl. Na+flux in the roots of seven-day-old seedlings was measured using the NMT technique described in the Methods section. (a) Changes in the NMT signals are expressed as arbitrary units. (b) Na+flux is expressed as the amount of efflux per second per square centimeter (pmol•cm− 2•s− 1). Data are presented as mean ± SE of three replicates. Same letter above the columns indicate that the differences at ap < 0.05 level among the different experimental cohorts are not significant statistically. SpSOS1,SpSOS1-overexpressing plants; SpAHA1,SpAHA1-overexpressing plants; SpSOS1-SpAHA1,SpSOS1andSpAHA1co-expressing plants; WT, wild-type plants. (TIF 456 kb)
Additional file 4:
Figure S4.Schematic of T-DNA region in the binary vectors. (a) The pCAMBIA1300-SpSOS1, (b) T pCAMBIA1304-SpSOS1, (c) pCAMBIA1304-SpSOS1-SpAHA1, and (d) pCAMBIA1304-SpAHA1plasmids. (TIF 239 kb)
Additional file 5:
Table S1.Sequences of primers used in this study. Small letters indicate restriction enzyme sites. (XLS 18 kb)
Rights and permissions
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fan, Y., Yin, X., Xie, Q.et al.Co-expression of SpSOS1 and SpAHA1 in transgenicArabidopsisplants improves salinity tolerance.BMCPlant Biol19,74 (2019). https://doi.org/10.1186/s12870-019-1680-7
Received:
Accepted:
Published:
Keywords
- H+-ATPase
- Na+/H+antiporter
- Plasma membrane
- Salt tolerance
- Sesuvium portulacastrum