- Research article
- Open Access
- Published:
一个排斥机制是一个内部的上位detoxification mechanism in aluminum resistance in Arabidopsis
BMC Plant Biologyvolume20, Article number:122(2020)
Abstract
Background
InArabidopsis, the aluminum (Al) exclusion mechanism is mainly facilitated by ALMT1-mediated malate exudation and MATE-mediated citrate releases from the root. Recently, we have demonstrated that coordinated functioning between an ALMT1-mediated Al exclusion mechanism, via exudation of malate from the root tip, and a NIP1;2-facilitated internal detoxification mechanism, via removal of Al from the root cell wall and subsequent root-to-shoot Al translocation, plays critical roles in achieving overall Al resistance. However, the genetic relationship betweenALMT1andNIP1;2in these processes remained unclear.
Results
Through genetic and physiological analyses, we demonstrate that unlikeALMT1andMATE, which function independently and additively,ALMT1andNIP1;2show an epistatic relationship in Al resistance. These results indicate thatALMT1andNIP1;2function in the same biochemical pathway, whereasALMT1andMATEin different ones.
Conclusion
The establishment of the epistatic relationship and the coordinated functioning between the ALMT1 and NIP1;2-mediated exclusion and internal detoxification mechanisms are pivotal for achieving overall Al resistance in the non-accumulating Arabidopsis plant. We discuss and emphasize the indispensable roles of the root cell wall for the implementation of the Al exclusion mechanism and for the establishment of an epistatic relationship between the ALMT1-mediated exclusion mechanism and the NIP1;2-facilitated internal detoxification mechanism.
Background
Aluminum (Al) is the most abundant metal element in the earth crust [1]. Under neutral or alkalescent conditions, Al is present in the soil as forms that are non-toxic to plants [2]. However, at low pH (< 5.0), aluminum ions (Al3+) are dissolved and released from the soil clays into the soil solutions, which could interact with multiple sites of the plant root cell, including the cell wall, cell membrane and cytosol with toxic effects, resulting severe root growth inhibition, the most significant symptom of Al toxicity [1,3]. The impaired root system restricts root absorption of water and nutrients from the acid subsoil, leading to drought stresses and nutrient deficiencies and thus reduced yields for crops grown on acidic soils [1,4].
Physiologic studies have indicated that the root apex, rather than the root elongation zone and the mature root region, is a major target of Al toxicity [5]. Cell wall loosening and synthesis of cell wall components are essential for sustained root cell elongation and water uptake [6,7]. However, Al3+ions severely inhibit root elongation through reducing cell wall cation binding, water permeability and cell wall enzyme activities [8,9,10,11]. As a result, the root cell wall in the root apical region is one of the major targets for Al toxicity [12,13].
Plants have adopted several strategies to cope with Al stresses, including Al exclusion and internal detoxification mechanisms [14,15]. The exclusion mechanism relies on root releases of chemical exudates, including organic acids [16], phenolic compounds [17], and phosphate [18], which facilitates the formation of non-toxic Al-exudate complexes in the rhizosphere and thereby prevents Al from entering the root cell, including the root apoplast [19]. Aluminum exclusion via release of organic-acid anions, including malate, citrate and oxalate, from the root apex is the best characterized, the most effective and widespread resistance mechanism employed by a large number of monocots and dicots plants [16,20,21,22,23,24,25,26,27,28]. Recently, Al- and salicylic acid (SA)-activated root exudation of benzoxazinoids has been recognized as an important exclusion mechanism for Al resistance in Maize [29].
The internal tolerance mechanism facilitates the detoxification of Al3+internally [30]. The processes include chelation of Al by organic acids in the cytosol, Al compartmentation in the vacuole of the root cell, translocation from sensitive root tissues to less sensitive shoot tissues where it is further sequestrated into the vacuoles of shoot cells [30,31,32]. However, functional and regulatory components underlying these processes remain largely unclear.
InArabidopsis thaliana, the exclusion mechanism plays a key role in Al resistance [24,25], which mainly relies on Al-activated root exudation of malate and citrate via the plasma membrane (PM)-localized malate transporter, ALMT1, and the citrate transporter MATE from the multidrug and toxic compound extrusion family, respectively [24,25]. ALMT1 facilitates the exudation of a large amount of malate from the root tip, while MATE mediates the release of a smaller amount of citrate from the more mature root region upon Al exposure [26]. The expression of bothALMT1andMATEis under the control of a master transcription factor, STOP1, i.e., sensitive to proton rhizotoxicity 1, which plays key roles in regulation of resistance to proton (low pH) and Al toxicity in plants [25,33,34].
InArabidopsis, Al3+ions in the rhizosphere can freely move and be retained in the root cell wall at low pH (< 5.0) [19]. The Al3+ions in the root cell wall directly or indirectly activate the PM-localized malate and citrate transporters, leading to organic acid releases from the cytosol of the root cell and formation of Al-organic acid complexes in the rhizosphere as well as in the root cell wall. Although it has been demonstrated that Al-organic acid complexes in the rhizosphere cannot enter the root cell, including the root cell wall [19], whether the Al-organic acid complexes retained in the root cell wall are toxic to the plant remained unclear previously.
Our recent studies have demonstrated that the Arabidopsis nodulin 26-like intrinsic protein 1;2 (NIP1;2) gene encodes a PM-localized transporter that specifically transports Al-malate (Al-Mal) complexes but not charged Al3+ions or other forms of Al-ligand complexes from the root cell wall into the root symplast [19,35]. As the transport substrate of NIP1;2 is the Al-Mal complex but not the Al3+ion, the ALMT1-mediated malate release into the root cell wall is a prerequisite for the NIP1;2-facilitated removal of Al from the root cell wall and subsequent translocation from the sensitive root tissues to the less sensitive shoot tissues [19]. Thus, the coordinated activities between the exclusion mechanism facilitated by ALMT1-mediated malate releases and the NIP1;2-mediated internal detoxification mechanism are essential for achieving overall Al tolerance in Arabidopsis [19,35].
在遗传学中,显性和隐性的used to describe the effects of different alleles at a genetic locus on determining the expression of a trait. Dominant alleles (AA) ultimately determine the expression of the trait, whereas recessive alleles (aa) are much less likely to be expressed. When a dominant allele is paired with a recessive one (Aa), the dominant allele (A) determines the trait. Recessive traits only manifest when both alleles in the locus are recessive in an individual (aa). In contrast, the term of epistasis is used to describe interactions between genes located in different genetic loci (e.g., A and B). It is referred to as a situation where the allelic actions of one locus (i.e., AA, Aa, or aa) mask the allelic effects of another locus (i.e., BB, Bb and bb), in the same way where the dominant allele mask the effects of the recessive one at the same locus [36,37]. In other words, epistasis describe a situation where the phenotypic expression at one locus depends on the genotype of a different locus.
Here, we provide further genetic evidence for the existence of an epistatic relationship betweenALMT1andNIP1;2. We demonstrate that such an epistatic relationship is required for orchestrating the functions of different Al resistance mechanisms in Arabidopsis. We emphasize the essential role of the root cell wall in establishing the epistatic relationship between the ALMT1-mediated exclusion mechanism and the NIP1;2-facilitated internal detoxification mechanism in Arabidopsis. We also discuss possible relationships between the exclusion and the internal detoxification mechanisms for Al accumulating plants under Al stresses.
Results
Generation of anamlt1_nip1;2double mutant line
Three independent T-DNA insertion mutants ofNIP1;2, i.e.,nip1;2–1(SALK_126593),nip1;2–2(SALK_147353) andnip1;2–3(SALK_076128), displayed comparable hypersensitive phenotypes to Al stresses at pH 4.3 (Additional file1: Figure S1) [19]. To further study the functional and genetic relationships betweenNIP1;2andALMT1, a homozygousalmt1_nip1;2double mutant line was generated through a cross betweenalmt1(SALK_009629) andnip1;2–3(hereafternip1;2), followed by selection from the F2 population of mutant plants with homozygousalmt1andnip1;2alleles.
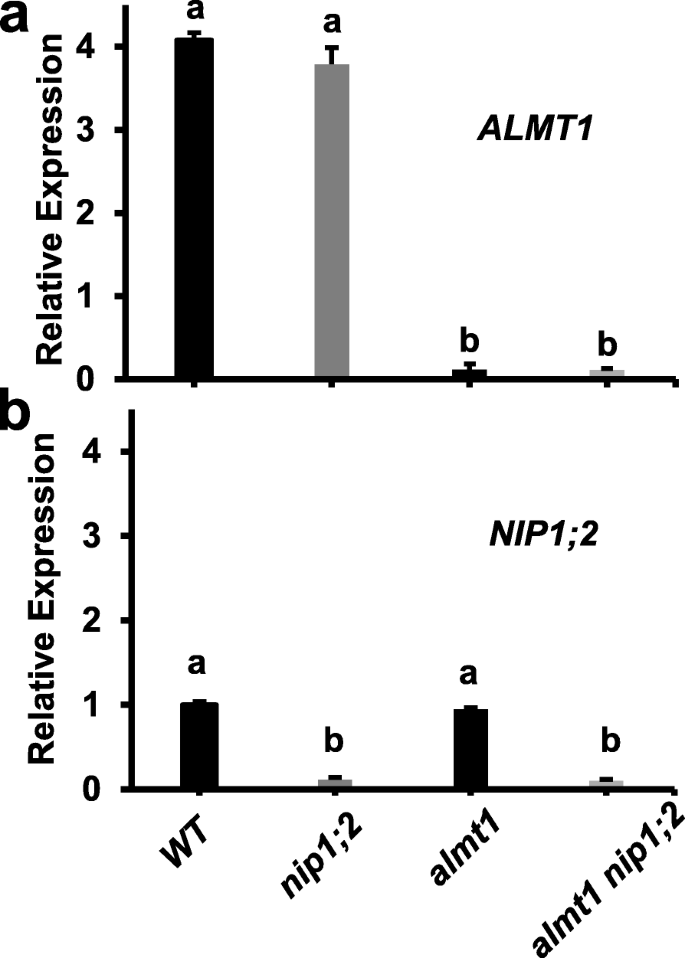
Real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analyses indicated that in the wild type (WT,Col-0), the expression ofALMT1andNIP1;2in the root was both induced by Al treatment although the levels ofALMT1transcripts were about 4-fold higher than those ofNIP1;2(Fig.1). Under thenip1;2mutant background, the level ofALMT1expression in the root was comparable with that in the WT (Fig.1a), whereasNIP1;2expression was greatly suppressed (Fig.1b). In contrast, under thealmt1background, although the level of theNIP1;2expression in the root were comparable with that in the WT, the Al-inducedALMT1expression in the root was barely detectable (Fig.1a). These results confirmed that bothalmt1andnip1;2are knockout (KO) mutants [19,24] and the expression ofALMT1andNIP1;2is independent of each other [35]. Under thealmt1_nip1;2background, the expression ofALMT1andNIP1;2in the root was both barely detectable (Fig.1a, b), indicating thatalmt1_nip1;2is a double KO mutant line.
Expression patterns ofALMT1(a) andNIP1;2(b) in the root. Roots of 7-day-old seedlings ofWT,almt1, nip1;2andalmt1_nip1;2treated with 20 μM AlCl3(pH 4.3) for 24 h were subject to RT-qPCR analysis. Data are means ± s.d. (n = 3); Different letters indicate significant differences between individual lines
Comparable sensitivity betweenalmt1_nip1;2andalmt1to Al stresses
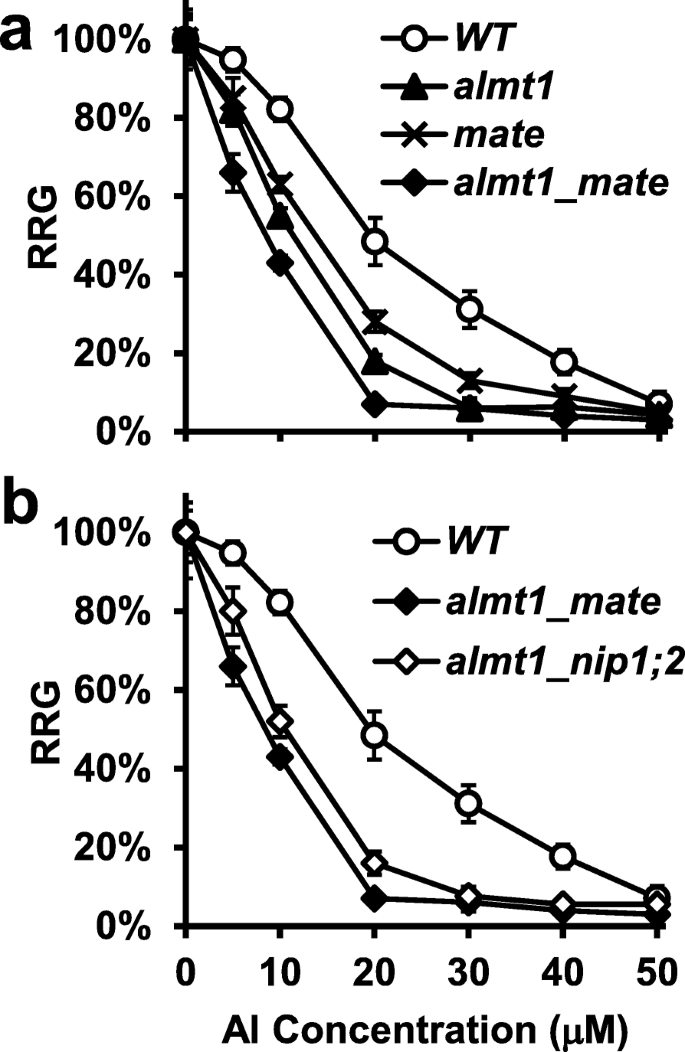
To evaluate Al resistance of individual lines, relative root growth (RRG%) was calculated for 5-day-old plants ofWT,nip1;2,almt1andalmt1_nip1;2treated with a range of Al concentrations (0–50 μM) at pH 4.3 (Fig.2). Root growth of both thealmt1andnip1;2单突变体被Al t更严重抑制han was the WT over the range of Al concentrations tested (Fig.2). Moreover, root growth was more strongly inhibited inalmt1than innip1;2(Fig.2). For instance, at Al concentration of 20 μM, root growth was inhibited by 82 and 69% inalmt1andnip1;2, respectively (Fig.2and Additional file1: Figure S2).
Relative root growth ofWTandalmt1, nip1;2andalmt1_nip1;2mutants. Seeds were germinated and grown in hydroponic solution (pH 4.3) supplemented with 0, 5, 10, 20, 30, 40, 50 μM of AlCl3for 5 days. Root length (mm) of individual seedlings was measured. Relative root growth (RRG%) was calculated according to the following formula: RRG% = root growth of individual plants under Al treatment/mean root growth under the control (−Al) condition. Data are means ± s.d. (n = 10)
In contrast, no significant differences in root growth were observed between thealmt1_nip1;2double mutant and thealmt1single mutant over the entire range of Al concentrations tested (Fig.2). Therefore, the Al-resistant phenotype ofalmt1_nip1;2resembled that ofalmt1, but notnip1;2.
Genetic analysis of allelic effects ofALMT1andNIP1;2on Al resistance
To evaluate the effects of genotypic variations at theALMT1andNIP1;2loci on Al resistance, a homozygousalmt1plant was crossed with a homozygousnip1;2plant to generate heterozygous F1 plants. The dominant and recessive alleles ofALMT1andNIP1;2would be segregated among the F2 plants.
Root growth was measured for F2 seedlings germinated and grown for 7 days in a hydroponic growth solution (pH 4.3) supplemented with 20 μM AlCl3. Based on their root growth, the F2 plants could be classified into three phenotypic groups (A, B and C) that showed significant differences in root growth (mm) under Al treatment (Table1and Additional file1: Figure S3). On the other hand, based on their allelic variations at theALMT1andNIP1;2loci, the F2 population could be classified into nine distinct genotypic groups/combinations (Table1and Additional file1: Figure S3).
Analysis of the relationship between phenotypic and genotypic variations indicated that1)the F2 plants in the phenotypic group A had at least one dominant wild-type allele at both theALMT1andNIP1;2loci (Table1and Additional file1: Figure S3);2)group B had homozygousalmt1/almt1alleles regardless of the status of theNIP1;2alleles; and3)group C had homozygousnip1;2/nip1;2mutant alleles and at least one wild-type allele ofALMT1, i.e.,almt1/ALMT1orALMT1/ALMT1.
In phenotypic group A, plants with at least one wild-typeALMT1allele and one wild-typeNIP1;2allele had comparable root growth under Al treatment as those with a wild-type background, i.e.,ALMT1/ALMT1 NIP1;2/NIP1;2(Table1and Additional file1: Figure S3). In contrast, homozygousalmt1and/ornip1;2plants in phenotypic groups B and C were more sensitive to Al compared with the plants in group A (Table1and Additional file1: Figure S3). These results indicated that the wild-type alleles ofALMT1andNIP1;2were both completely dominant.
Although homozygous mutations ofalmt1ornip1;2caused significant root growth inhibition (Fig.2and Table1), the effects of genotypic variation at one locus on the phenotypic expression of the other locus were quite different betweenALMT1andNIP1;2(Table1). For instance, under a homozygousalmt1/almt1background (group B, Table1), root growth was solely determined by the homozygousalmt1mutant alleles regardless of the genotypic variation at theNIP1;2locus (Table1and Additional file1: Figure S3). In contrast, under a homozygousnip1;2/nip1;2background, the degrees of root growth inhibition by Al were strongly affected by the genotypic variation at theALMT1locus. For instance, plants with homozygousalmt1/almt1alleles, i.e., thealmt1_nip1;2double mutant plants, displayed greatly enhanced root-growth inhibition compared with those with one or two copies of the wild-typeALMT1allele in group C (Table1and Additional file1: Figure S3). The fact that the homozygousalmt1mutation at theALMT1轨迹可以掩盖/覆盖基因型的影响variation at theNIP1;2locus indicates that there exist interactions between theALMT1andNIP1;2loci whereALMT1is genetically epistatic toNIP1;2.
Additive effects ofALMT1andMATEand epistatic relationship betweenALMT1andNIP1;2in Al resistance
In Arabidopsis, the Al-activated and ALMT1-facilated malate exudation from the root-tip region plays a major role in Al resistance, while the Al-activated and MATE-mediated citrate release from more mature root regions plays a smaller but significant role [24,25,26]. Although both thealmt1andmatesingle mutants were more sensitive to a range of Al concentrations (0–50 μM) tested than was the WT,almt1consistently displayed significantly stronger root-growth inhibition than did thematemutant (Fig.3a and Additional file1: Figure S2).
Compared with thealmt1andmatesingle mutants, thealmt1_matedouble mutant showed significantly more severe root-growth inhibition phenotypes over the entire range of Al concentrations tested (Fig.3a and Additional file1: Figure S2). For instance, at 20 μM Al, root growth ofalmt1_matewas inhibited by 93%, whereas root growth ofalmt1andmateby 82 and 72%, respectively (Fig.3a). Thus, the effects ofALMT1andMATEon Al resistance are additive, suggesting that ALMT1 and MATE function in different biochemical pathways, which is consistent with our previous observation that ALMT1 and MATE function independently in achieving overall Al resistance in Arabidopsis [25].
In contrast, thealmt1_nip1;2double mutant did not display stronger mutant phenotypes than did thealmt1single mutant (Fig.2). Instead, root growth was comparable betweenalmt1andalmt1_nip1;2over the entire range of Al concentrations tested (Fig.2).
ALMT1-mediated root exudation of malate is independent of NIP1;2 function
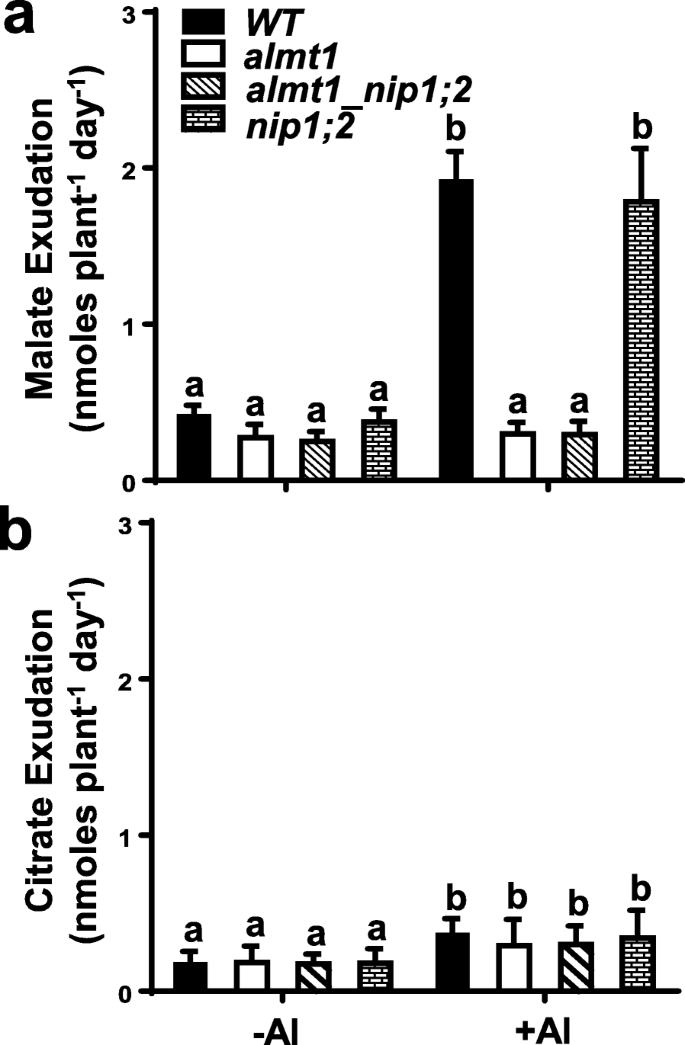
评估不同基因型的影响root organic acid exudation, Al-activated root exudation of malate and citrate was examined forWT,almt1,nip1;2andalmt1_nip1;2.Under the control condition (−Al), comparable basal levels of root exudation of malate and citrate were observed among individual lines (Fig.4a, b). Al exposure triggered releases of large and comparable amounts of malate from the roots ofWTand thenip1;2mutant (Fig.4a). In contrast, bothalmt1andalmt1_nip1;2缺乏可检测Al-activated根酸盐exudation (Fig.4a). These results indicate that Al-activated malate exudation from the root is mainly facilitated by the ALMT1 malate transporter in Arabidopsis and the Al-activated and ALMT1-mediated root malate exudation is independent of the NIP1;2 function.
Root exudation of malate (a) and citrate (b). Here, 6-day-old seedlings ofWT,almt1, nip1;2andalmt1nip1;2were treated in 20 ml of exudation buffers (pH 4.3) supplemented without (−) or with (+) 50 μM AlCl3for 2 days before the concentrations of malate and citrate in the exudation buffer were determined. Data are means ± s.d. of three biological replicates.Lettersrepresent groups with significant differences (P ≤ 0.05)
Compared with root malate exudation, Al exposure also triggered smaller, but significant, increases in citrate exudation from the root (Fig.4b). In contrast, no significant differences were observed in the amounts of citrate in the root exudates from all lines examined upon Al exposure (Fig.4b). These results indicate that the Al-activated and MATE-facilitated root citrate exudation is independent of the ALMT1 and NIP1;2 functions in Arabidopsis (Fig.4b). Thus, the phenotypes of organic acid exudation of thealmt1_nip1;2double KO line resemble those of thealmt1mutant, but not thenip1;2mutant.
ALMT1 functions upstream of NIP1;2 in the process of Al removal from the root cell wall
To test the relationship betweenALMT1andNIP1;2in the processes of Al removal from the root cell wall, Al contents in the root cell wall and cell sap were measured for theWT,almt1,nip1;2andalmt1_nip1;2plants treated with 50 μM AlCl3at pH 4.3 for 2 d (Fig.5). Compared with theWTplants, thealmt1andnip1;2plants accumulated significantly higher and lower concentrations of Al in the root cell walls (Fig.5a) and root cell sap (Fig.5b), respectively. These results confirmed that both ALMT1-mediated malate releases and a functional NIP1;2 are required for Al removal from the root cell wall into the root cytosol [19]. However, thealmt1mutant also accumulated significantly higher and lower concentrations of Al in the root cell wall (Fig.5a) and the root cell sap (Fig.5b), respectively, compared with thenip1;2mutant. These results suggest that ALMT1-depenedent but NIP1;2-indenpent processes are present for Al removal from the root cell wall in Arabidopsis.
Aluminum content in the root cell wall (a) and the root cell sap (b). Seven-day-old seedlings of WT,almt1, nip1;2andalmt1_nip1;2were exposed to 50 μM AlCl3(pH 4.3) for 2 days. Al concentrations in the root cell wall (a) and the root cell sap (b) were determined by ICP-MS. Data are mean ± s.d. of three biological replicates from three Magenta boxes.Lettersrepresent groups with significant differences (P ≤ 0.05)
The Al concentrations in the root cell wall (Fig.5a) and root cell sap of thealmt1_nip1;2double mutant (Fig.5b) were comparable with those of thealmt1single mutant, which were significantly different from those in thenip1;2single mutant. These results indicate thatALMT1is genetically epistatic toNIP1;2in the biochemical pathway leading to Al removal from the root cell wall into the root symplasm in Arabidopsis.
Externally supplied malate partially restored NIP1;2-facilitated Al uptakes from the root cell wall inalmt1but not inalmt1_nip1;2
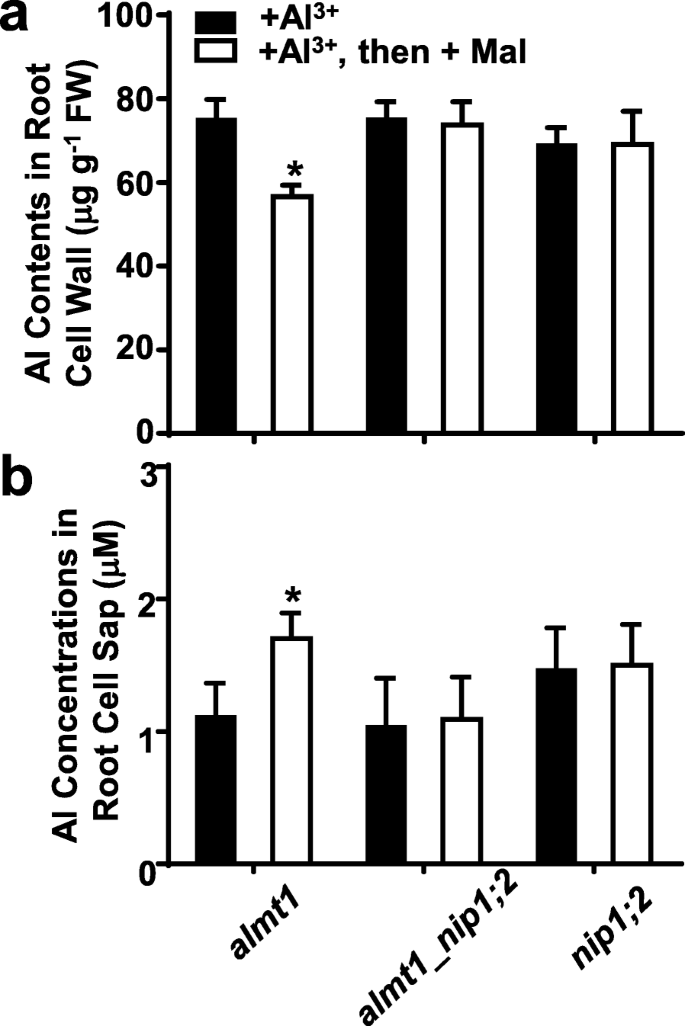
To evaluate the effects of externally supplied malate on Al uptakes from the root cell wall foralmt1,nip1;2andalmt1_nip1;2, plants were treated with 50 μM AlCl3(pH 4.3) for 8 h, allowing Al to get into and be retained in the root cell walls (Fig.6a) [19], followed by addition of 0 or 200 μM malate for another 8 h.
Effects of externally supplied malate in NIP1;2-mediated Al uptake in thealmt1, almt1_nip1;2andnip1;2lines. Here, 7-day-old seedlings were pretreated with AlCl3(pH 4.3) for 8 h, washed three times with 0.5 mM CaCl2, and then treated with 200 μM malate (−Al) for 8 h.aAl concentrations in the root cell wall andbroot cell sap were determined by ICP-MS. Data are mean ± s.d. of three sample replicates from three Magenta boxes. *, significant differences (P ≤ 0.05) between the – and the + malate treatment
Between these two treatments, no statistically significant differences in Al contents in the root cell wall (Fig.6a) and the root cell sap (Fig.6b) were observed in thenip1;2single mutant and thealmt1_nip1;2double mutant. In contrast, inalmt1, compared with those under the Al treatment alone, external supplementation of malate after Al treatment led to significantly decreased Al concentrations in the root cell wall (Fig.6a) and significantly increased concentrations in the root cell sap (Fig.6b). These results indicate that even though thealmt1mutant has a functional NIP1;2 transporter [19], the presence of malate in the root cell wall is essential for NIP1;2-facilitated Al removals from the root cell wall. Thus, the ALMT1-mediated releases of malate to the root cell wall function in an earlier step in the NIP1;2-facilitated process for Al uptakes from the root cell wall to the root cytosol.
Discussion
ALMT1is genetically and functionally epistatic toNIP1;2
In Arabidopsis, coordinated activity of ALMT1 and NIP1;2 is required for NIP1;2-facilitated Al removal from the root cell wall into the root symplasm and subsequent root-to-shoot Al translocation, which are critical steps in the internal detoxification mechanism [19,32,35]. In this process, first, the Al3+ions enter the root apoplast from the rhizosphere and then activate the PM-localized ALMT1 transporter, leading to malate exudation from the root tip cell into the root apoplast and rhizosphere [24,25,26]. In the root apoplast, the released malate interacts with the Al3+ions to form Al-Mal complexes, which are subsequently transported from the root cell wall into the root cytosol by the PM-localized NIP1;2 [19]. As NIP1;2 transports the Al-Mal complex, but not the Al3+ion, the ALMT1-mediated malate exudation into the root cell wall is required for the formation of Al-Mal complexes in the root apoplast and the subsequent NIP1;2-facilitated Al removal from the root cell wall (Fig.5) [19]. Thus, ALMT1 plays a key role in both the exclusion mechanism, via facilitating malate exudation to the rhizosphere to chelate toxic Al3+ions, and the internal detoxification mechanism, through facilitating the NIP1;2-mediated removal of the Al-Mal complex from the root cell wall and translocation from the root to the shoot [19].
Phenotypic examination of root growth indicated that thealmt1_matedouble mutant was more sensitive to Al than was either of thealmt1ormatesingle mutants, indicating that the effects ofalmt1andmatemutations are additive (Fig.3a). In contrast, no additive or synergistic effects were observed between thealmt1andnip1;2mutations (Fig.2and Additional file1: Figure S1). Therefore, our root-growth experimental conditions could distinguish the additive effect ofALMT1andMATEfrom the epistatic relationship betweenALMT1andNIP1;2. Further examination of Al-activated root organic-acid exudation (Fig.4) and NIP1;2-facilitated Al removal from the root cell wall (Fig.5)也表明的表型almt1_nip1;2double KO mutant resembled those ofalmt1, but notnip1;2. Moreover, externally supplied malate could partially compensate the loss of the ALMT1-mediated malate exudation for the NIP1;2-facilitated function in thealmt1mutant (Fig.6). Taken together, these results indicate that ALMT1 and NIP1;2 function in a single biochemical pathway, where ALMT1 functions upstream of NIP1;2. The fact that thealmt1mutant is more sensitive to Al toxicity than was thenip1;2mutant (Fig.2) also indicates thatALMT1plays a larger role in contribution to overall Al resistance than doesNIP1;2in Arabidopsis.
The significance of the epistatic relationship between ALMT1 and NIP1;2 in Al tolerance for the non-accumulating Arabidopsis
On acid soils, most plants, i.e., the so-called non-accumulators, limit the uptake of Al from the soil and accumulate no more than 0.2 mg Al g− 1dry weight of the plant [32]. In contrast, a few Al accumulator plant species can accumulate much higher concentrations of Al in the shoot/leaf. For instance, hydrangea (Hydrangea macrophylla) plants can accumulate up to 3 mg Al g− 1dry weight of the plant [32], while buckwheat (Fagopyrum esculentum) plants 1.7 mg Al g− 1dry weight without showing any sings of toxicity [38,39].
In both the accumulating and non-accumulating plant species, overall Al resistance can be achieved by the exclusion and the internal tolerance mechanisms [15,32,40]. However, these two mechanisms are not mutually exclusive but they are coordinately functioned as in the case of the ALMT1- and NIP1;2-mediated Al tolerance in Arabidopsis [19,35].
Arabidopsis is a non-accumulating species for Al. Therefore, Al tolerance in Arabidopsis is mainly dependent on the exclusion mechanism via the ALMT1- and MATE-mediated root exudation of malate and citrate, respectively, to the rhizosphere and the root apoplast where organic acids chelate the Al3+ions [24,25,26]. As ALMT1 facilitates the release of a large amount of malate from the root tip region [26], a major target for Al toxicity [12,13,41,42], ALMT1 makes a significantly larger contribution to overall Al resistance in Arabidopsis than MATE [25,26].
In the root apoplast, the released malate anions chelate the Al3+cations and thus reduce the concentration of free Al3+cations, which minimizes the harmful interactions of these cations in the cell wall. However, the simple binding of Al and malate in the apoplast of the root cell is not enough to provide full protection against Al toxicity [19]. Instead, the Al-Mal complexes in the root apoplast need to be removed for achieving higher degrees of Al tolerance [19].
Here, we further demonstrate that ALMT1 is genetically and functionally epistatic to NIP1;2. Such an epistatic relationship allows the Arabidopsis plant to be protected by the exclusion mechanism first, which effectively blocks the entry of toxic Al3+cations into the root cell, including the root cell wall, before the NIP1;2-facilitated internal detoxification mechanism, i.e., uptakes of Al from the root cell wall, starts to function. Without the establishment of such an epistatic relationship between the exclusion and internal detoxification mechanisms, high levels of Al could be accumulated in the plant, which is harmful to the non-accumulator like Arabidopsis.
Therefore, for the non-accumulating plant species, a dominant exclusion mechanism is essential for Al resistance via continuously preventing the entry of Al to the root cell. After the exclusion mechanism is established, the internal detoxification mechanism will step in and play a secondary and scavenging role for removing the toxic Al3+ions from the root apoplast and cytosol for further sequestration into the vacuole of the root cell and/or for translocation from the root to less sensitive shoot.
In contrast, for the accumulating plants to take up large amounts of Al3+ions from the root and accumulate them in the shoot, the exclusion mechanism must be suppressed. Interestingly, Al-activated release of oxalic acid is required for the protection of root growth and function for young seedlings of buckwheat, an Al accumulator, under Al stresses [43]. Thus, it is likely that at the early developmental stage, the exclusion mechanism is also essential for Al accumulators to withstand the initial shocks of Al toxicity. Once the internal detoxification mechanism is established at later developmental stages, the exclusion mechanism will be suppressed or discontinued to function, allowing the accumulation of large amounts of Al in the shoot via the internal detoxification mechanism.
Essential roles of the root cell wall in overall Al resistance in Arabidopsis
As mentioned above, the significance of the epistatic relationship between the exclusion and internal detoxification mechanisms in Arabidopsis lies in that the ALMT1-mediated exclusion mechanism provides a shield/barrier for the root against the toxic Al3+ions in the rhizosphere before the NIP1;2-facilitated internal detoxification mechanism is allowed to function. However, the pivotal roles of the root cell wall are less well recognized in this system.
The root cell wall is highly negatively charged and as a result, Al3+cations in the rhizosphere can freely enter and be retained in the root apoplast at low pH (< 5.0) [19]. The Al-activated and ALMT1-mediated malate release to the rhizosphere results in the formation of Al-Mal complexes in the rhizosphere. As the Al-Mal complex is unable to enter the root cell wall [19], with a functional ALMT1-mediated malate exudation to the rhizosphere, it is the root cell wall that acts as a shield/barrier that separates the Al in the rhizosphere from the root symplasm. Furthermore, the presence of such a shield ensures that only the Al-Mal complex in the root cell wall, but not in the rhizosphere, is accessible to the NIP1;2 transporter localized to the PM of the root cell. In conclusion, the root cell wall plays pivotal roles in the establishment of the exclusion mechanism as well as the epistatic relationship between the exclusion and the internal detoxification mechanisms for overall Al resistance and for the prevention of over-accumulation of Al in Arabidopsis plants.
Conclusion
Here, we demonstrate that ALMT1 is genetically epistatic to NIP1;2 for achieving coordinated functions between an exclusion and an internal tolerance mechanism and overall Al resistance in the non-accumulating Arabidopsis plant. This elegant system ensures that an exclusion mechanism is established before an internal tolerance mechanism steps in to achieve overall Al resistance in the non-accumulating Arabidopsis. The root cell wall plays indispensable roles in implementation of the Al exclusion mechanism and the establishment of an epistatic relationship between the ALMT1-mediated exclusion mechanism and the NIP1;2-facilitated internal detoxification mechanism. These findings further expand our knowledge about how overall Al resistance is achieved in plants.
Methods
Materials and culture conditions
Arabidopsis T-DNA insertion linesnip1;2–1(SALK_126593),nip1;2–2(SALK_147353),nip1;2–3(SALK_076128),mate(SALK_081671) andalmt1(SALK_009629C) as well as WT (Col-0; CS60006) were acquired from the Arabidopsis Biological Resource Center (https://abrc.osu.edu/). Thealmt1_nip1;2double mutant was generated by crossingalmt1andnip1;2–3(nip1;2) single mutants, followed by selection of F2 plants with homozygous T-DNA insertions at both theALMT1andNIP1;2loci by PCR-based genotyping [19]. Thealmt1_matedouble mutant was generated previously [25].
Primer sequences for genotyping theALMT1locus were 5′-CGCAGCTGCACATATATCACA-3′ (ALMT1gene specific primer) and 5′-GCTGTTGCCCGTCTCACTGGTG-3′ (T-DNA left border primer) for detection of the T-DNA insertion; and 5′-CGCAGCTGCACATATATCACA-3′ and 5′-CGAAGTGCAACGCACCACTA-3′ for amplification of the sequence encompassing the T-DNA insertion region. Primer sequences for genotyping theNIP1;2locus fornip1;2–3were 5′-GCTCGCATCTAGATCCTAAT-3′ (NIP1;2gene specific primer) and 5′-GCTGTTGCCCGTCTCACTGGTG-3′ (T-DNA left border primer) for detection of the T-DNA insertion; 5′- GCTCGCATCTAGATCCTAAT-3′ and 5′-CGAAGTGCAACGCACCACTA-3′ for amplification of the sequence encompassing the T-DNA insertion region. The positions of the T-DNA insertions as well as the primers listed above were depicted in Additional file1: Figure S4.
The PCR mixture (25 μL) contains the following reagents: 100 ng gDNA template, 1X Green GoTaq® Reaction Buffer (1.5 mM MgCl2) (Promega), 0.2 mM each dNTP, 0.5 μM upstream primer, 0.5 μM downstream primer, GoTaq DNA polymerase (Promega). The PCR reactions were conducted with a T100 Thermal Cycler (BioRad) with the following thermal cycling conditions: 1 cycle of 95 °C for 2 min; 25 cycles of 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 2 min; final extension at 72 °C for 5 min.
For growth experiments, seeds of the wild type, individual mutant lines or the F2 population from the cross ofalmt1andnip1;2were surface-sterilized and cold stratified at 4 °C in the dark for 3 days for synchronization of germination. Seeds were subsequently sown onto a 250 μM polypropylene mesh in a Magenta box containing a hydroponic growth solution, supplemented with 2.0 mM Homo-PIPES to maintain pH at 4.3. The hydroponic solution consisted of the following macronutrients in mM: MgCl2, 3.0; (NH4)2SO4, 0.25; Ca (NO3)2, 1.0 M; KCl, 2.0; CaCl2, 2.75; KH2P04, 0.18; and the following micronutrients in μM: H3BO3, 50.0; MnSO4, 10.0; CuSO4, 0.5; ZnSO4, 2.0; Na2MoO4, 0.1; CoCl2, 0.1; 1% sucrose. Plants were grown in a growth chamber (Pervival, Model I-36LLVL) with 23 °C temperature, 65% humidity and light intensity of 100 μmol photons m2/s by cool-white fluorescent tubes (GE) and 16-h photoperiod.
For evaluating Al sensitivity, seeds of the WT and individual mutant lines, i.e.,nip1;2,almt1,almt1_mateandalmt1_nip1;2, were germinated and grown in the above-mentioned hydroponic solution (pH 4.3) supplemented with 0, 5, 10, 20, 30, 40 or 50 μM of AlCl3for 7 days. Relative root growth (RRG%) was calculated according to the following formula: RRG% = root growth (mm) of individual plants under Al treatment/mean root growth (mm) under the control (−Al) condition.
For phenotyping and genotyping the F2 individuals derived from the cross ofalmt1andnip1;2, F2 seeds were germinated and grown in the hydroponic solution (pH 4.3) supplemented with 20 μM AlCl3in Magenta boxes (~ 120 seed in each box) for 7 days. Root length (mm) of 215 randomly selected plants was measured before the plants were transferred to the soil for growth for 2 weeks. Then, DNAs were extracted from leaves of individual F2 plants. T-DNA insertions at theALMT1andNIP1;2loci were evaluated by PCR followed the procedures mentioned above. Based on the genotypes at theALMT1andNIP1;2loci, the F2 population could be classified into nine distinct genotypic combinations/groups (Table1; Additional file1: Figure S3). Ten plants were randomly selected from each of the nine genotypic groups for calculation of the mean root growth (mm) of the group (Table1; Additional file1: Figure S3). The root growth data were also used for performing Fisher’s least significant difference (LSD) tests to distinguish statistically different phenotypic groups in the F2 population (Table1; Additional file1: Figure S3).
RNA isolation and real-time RT-qPCR
For gene expression analysis, ~ 500 seeds (~ 10 mg) were germinated in the above-mentioned control hydroponic solution (−Al) in a Magenta box for 6 days. Then, seedlings were transferred to a fresh hydroponic solution (pH 4.3) containing 20 μM AlCl3and treated for 24 h before the root samples were collected. Three replicates (Magenta boxes) were included for each of theWT,nip1;2,almt1andalmt1_nip1;2lines.
Total RNAs were extracted from the roots using an RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions. First-strand cDNA was synthesized in a reaction cocktail containing 1X reaction buffer, 5 μg DNaseI-digested total RNA, 2.5 μM of random oligos, 1 mM of each dNTP, 5 μL of SuperScript III reverse transcriptase (Thermal Scientific, Inc.) in a total volume of 100 μL. The reaction was performed at 37 °C for 90 min, followed by heating at 72 °C for 10 min. Subsequently, 2 μL of RNase H (Thermal Scientific, Inc.) was added to each RT sample for 1.5-h incubation at 37 °C. The synthesized samples were stored at -20 °C until use.
Real-time RT-qPCR was performed on a 7500 Fast Real-Time PCR System (Thermal Scientific, Inc.). Concentrations of each of cDNA samples were adjusted to 1 μg/μL. Each real-time PCR reaction contained 2 μL of diluted cDNA sample, 10 μL of 2X Power SYBR Green PCR Master Mix (Thermal Scientific, Inc.), 0.15 μM primer (forward and reverse each) in 20 μl reaction volume. Three technical replicates were included for each cDNA sample. The real-time PCR cycling conditions were 95 °C for 3 min; 39 cycles of 95 °C for 10 s, 60 °C for 30 s, plate reading; 1 cycle of 65 °C for 30 s; 60 cycles of 65 °C for 5 s (+ 0.5 °C/cycle, ramp 0.5 °C/sec), plate reading.
The sequences of optimal gene-specific real-time RT-qPCR primers wereNIP1;2, 5′- GGTTCGATATACTGATAAGCCA-3′ and 5′-GATACAACTTAACCTCCGATGAC-3′ (137 bp amplicon);ALMT1, 5′-TTCCCGATTCCGAGCTCATT-3′ (located in exon 5 and exon 6 junction) and 5′-CTCAGATTTTCAGATCCCAGTGGAC-3′ (80 bp amplicon);18SrRNA (endogenous calibrator gene), 5′-CGCTATTGGAGCTGGAATTACC-3′, 5′-AATCCCTTAACGAGGATCCATTG-3′ (71 bp amplicon). Gene structure, locations of the real-time PCR primers for corresponding genes were depicted (Additional file1: Figure S5). In all real-time PCR amplifications mentioned below, a single peak of the dissociation curve was observed for each of the real-time RT-qPCR primer pairs, indicating that the primers were highly specific for the target genes.
To construct standard curves for theNIP1;2,ALMT1and18S rRNAamplifications, a serial dilutions (5x) of a cDNA sample (1 μg/μl) prepared from total WT RNA were prepared and subject to real-time PCR thermal cycling as mentioned above. Standard curves for the target genes (ALMT1andNIP1;2) and the endogenous control gene (18S rRNA) were plotted as the log ng cDNA (six logs or dilutions included, three technical replicates for each dilution) vs. CTvalues of the corresponding samples. The equation for the standard curves was y = mx + b, where y was CT, x was log ng of the cDNA sample, m the slope of standard curve line and b the y-intercept of the standard line. For the standard curves of theNIP1;2,ALMT1and18S rRNAamplification tested, m was ~ − 3.3 and R2 > 0.99, indicating high efficiency of the primers for PCR amplification.
Real-time qPCR samples for testing the expression of a known gene (i.e.,ALMT1,NIP1;2or18S rRNA) were put in a same 384-well plate together with the standard curve samples of the corresponding gene. The qPCR thermal cycling conditions were as mentioned above. The quantity of the real-time qPCR amplicons of the known gene were calculated for each sample based on its CT value and the standard curve of the corresponding known gene. Relative gene expression was calculated as the quantity of the target genes (i.e.,ALMT1orNIP1;2) divided by the quantity of the18S rRNAgene of the same cDNA sample.
Detection of organic acid exudation from roots
Surface-sterilized seeds (~ 2–3 mg) from each line were germinated in Magenta boxes containing the sterile hydroponic growth solution (pH 4.3) for 6 days, and then the seedlings were transferred to 20 ml of filter-sterilized exudation solutions (pH 4.3) with or without 50 μM Al3+in a sterile Petri dish for 2 days. The exudation solution consisted of the following macronutrients in μM: MgCl2, 275; CaCl2, 275; KCl, 275; Ca (NO3)2, 33.4; MgSO4, 33.4; K2SO4,16.7; and the following micronutrients in μM: H3BO3, 50.0; MnSO4, 10.0; CuSO4, 0.5; ZnSO4, 2.0; Na2MoO4, 0.1; CoCl2, 0.1; and 1% sucrose, supplemented with 3.0 mM Homo-PIPES (pH 4.3). Then, the exudation solutions were collected and the numbers of plants were counted. To remove Al3+and other inorganic anions, the exudation solutions were treated with anionic and cationic chromatography columns. Subsequently, the eluate was concentrated to dryness using a rotary evaporator at 40 °C. The residue was re-dissolved in 1 ml of Milli-Q water. Malate and citrate concentrations were then measured according to the enzymatic method previously described [22].
Root cell sap and cell wall preparation and Al determination
Arabidopsis lines were firstly germinated and grown in the hydroponic solution (pH 4.3) for 7 days, then treated in a fresh hydroponic solution (pH 4.3) supplemented with 50 μM AlCl3for 2 d. After the treatment, the roots were cut and washed three times with deionized water. The cut root samples were centrifuged at 3000 rpm for 10 min at 4 °C in an Ultra free-MC Centrifugal filter unit (Millipore) to remove the apoplastic solution, and frozen in a − 80 °C freezer overnight. The frozen root samples were de-frozen at room temperature, and then centrifuging at 13,000 rpm for 10 min to separate the root cell sap solution from the residual cell wall. The cell wall sample was washed with 70% ethanol three times and then digested in 1 mL of 2 M HCl for at least 24 h with gentle shaking. Al contents in the symplastic solution and cell wall extract were determined by inductively coupled plasma mass spectrometry (ICP-MS).
For testing the effects of sequential Al3+and malate treatment on Al accumulation, ~ 150 7-d-old seedlings of the WT,almt1, nip1;2–3andalmt1_nip1;2lines were pretreated with the hydroponic solution (pH 4.3) supplemented with 50 μM AlCl3for 8 h. The samples were then washed three times with 0.5 mM CaCl2and treated in hydroponic solutions (pH 4.3) supplemented with or without 200 μM malate for 8 h. Aluminum concentrations in cell sap and cell wall were measured as mentioned above. Three biological replicates (Magenta boxes) with the same setting were prepared for each plant line and each treatment.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Al-Mal:
-
Aluminum-malate
- ALMT:
-
Al-activated malate transporter
- KO:
-
Knockout
- MATE:
-
Multidrug and toxic compound extrusion
- NIP:
-
Nodulin 26-like intrinsic protein
- RRG:
-
Relative root growth
References
- 1.
Kochian LV, Hoekenga OA, Pineros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol. 2004;55:459–93.
- 2.
Zhang X, Long Y, Huang J, Xia J. Molecular mechanisms for coping with Al toxicity in plants. Int J Mol Sci. 2019;20:1551.
- 3.
Rout G, Samantaray S, Das P. Aluminium toxicity in plants: a review. Agronomie. 2001;21:3–21.
- 4.
Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol. 2000;200:1–46.
- 5.
Ryan PR, Ditomaso JM, Kochian LV. Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot. 1993;44:437–46.
- 6.
McQueen-Mason S, Rochange F. Expansins in plant growth and development: an update on an emerging topic. Plant Biol. 1999;1:19–25.
- 7.
Voesenek L, Benschop J, Bou J, Cox M, Groeneveld H, Millenaar F, Vreeburg R, Peeters A. Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris. Ann Bot. 2003;91:205–11.
- 8.
Gunse B, Poschenrieder C, Barcelo J. Water transport properties of roots and root cortical cells in proton-and Al-stressed maize varieties. Plant Physiol. 1997;113:595–602.
- 9.
Ma JF, Shen R, Nagao S, Tanimoto E. Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol. 2004;45:583–9.
- 10.
Tabuchi A, Matsumoto H. Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiol Plantarum. 2001;112:353–8.
- 11.
Horst WJ. The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Z Pflanzenernähr Bodenkd. 1995;158:419–28.
- 12.
Horst WJ, Wang Y, Eticha D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot. 2010;106:185–97.
- 13.
他C,杨azbzcx,耿X张F,王R,霍斯特WJ,Ding Z. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell. 2014;26:2889–904.
- 14.
刘J,马皮尼罗,Kochian LV。aluminu的作用m sensing and signaling in plant aluminum resistance. J Integr Plant Biol. 2014;56:221–30.
- 15.
Kochian LV, Piñeros M, Liu J, Magalhaes J. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol. 2015;66:23–8.
- 16.
Ma JF, Ryan PR, Delhaize E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001;6:273–8.
- 17.
Tolrà R, Barceló J, Poschenrieder C. Constitutive and aluminium-induced patterns of phenolic compounds in two maize varieties differing in aluminium tolerance. J Inorg Biochem. 2009;103:1486–90.
- 18.
Pellet DM, Papernik LA, Kochian LV. Multiple aluminum-resistance mechanisms in wheat (roles of root apical phosphate and malate exudation). Plant Physiol. 1996;112:591–7.
- 19.
Wang Y, Li R, Li D, Jia X, Zhou D, Li J, Lyi SM, Hou S, Huang Y, Kochian LV, Liu J. NIP1;2 is a plasma membrane-localized transporter mediating aluminum uptake, translocation, and tolerance in Arabidopsis. Proc Natl Acad Sci U S A. 2017;114(19):5047–52.
- 20.
Qiu W, Wang N, Dai J, Wang T, Kochian LV, Liu J, Zuo Y. AhFRDL1 mediated citrate secretion contributes to adaptation of peanuts for Fe deficiency and Al stress. J Exp Bot. 2019;70:2873–86.
- 21.
Melo JO, Lana UG, Piñeros MA, Alves V, Guimarães CT, Liu J, Zheng Y, Zhong S, Fei Z, Maron LG. Incomplete transfer of accessory loci influencing SbMATE expression underlies genetic background effects for aluminum tolerance in sorghum. Plant J. 2013;73:276–88.
- 22.
Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivumL.)(II. Aluminum-stimulated excretion of malic acid from root apices). Plant Physiol. 1993;103:695–702.
- 23.
Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF. An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007;48:1081–91.
- 24.
Hoekenga OA, Maron LG, Piñeros MA, Cançado GM, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T.AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci U S A. 2006;103:9738–43.
- 25.
Liu J, Magalhaes JV, Shaff J, Kochian LV. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009;57:389–99.
- 26.
Liu J, Luo X, Shaff J, Liang C, Jia X, Li Z, Magalhaes J, Kochian LV. A promoter-swap strategy between theAtALMTandAtMATEgenes increased Arabidopsis aluminum resistance and improved carbon-use efficiency for aluminum resistance. Plant J. 2012;71:327–37.
- 27.
Magalhaes合资,刘J,吉马良斯CT,拉娜UG,阿尔维斯VM, Wang Y-H, Schaffert RE, Hoekenga OA, Pineros MA, Shaff JE. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet. 2007;39:1156–61.
- 28.
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004;37:645–53.
- 29.
Zhao Z, Gao X, Ke Y, Chang M, Xie L, Li X, Gu M, Liu J, Tang X. A unique aluminum resistance mechanism conferred by aluminum and salicylic-acid-activated root efflux of benzoxazinoids in maize. Plant Soil. 2019;437:273–89.
- 30.
Delhaize E, Gruber BD, Ryan PR. The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett. 2007;581:2255–62.
- 31.
Ma JF, Hiradate S, Matsumoto H. High aluminum resistance in buckwheat II. Oxalic acid detoxifies aluminum internally. Plant Physiol. 1998;117:753–9.
- 32.
Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H. Internal detoxification mechanism of Al in hydrangea (identification of Al form in the leaves). Plant Physiol. 1997;113:1033–9.
- 33.
Iuchi S, Koyama H, Iuchi A, Kobayashi Y, Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci U S A. 2007;104:9900–5.
- 34.
Jiang F, Wang T, Wang Y, Kochian LV, Chen F, Liu J. Identification and characterization of suppressor mutants ofstop1. BMC Plant Biol. 2017;17:128.
- 35.
Wang Y, Cai Y, Cao Y, Liu J. Aluminum-activated root malate and citrate exudation is independent of NIP1;2-facilitated root-cell-wall aluminum removal in Arabidopsis. Plant Signal Behav. 2018;13:e1422469.
- 36.
Cordell HJ. Epistasis: what it means, what it doesn't mean, and statistical methods to detect it in humans. Human Mol Genet. 2002;11:2463–8.
- 37.
Phillips PC. Epistasis—the essential role of gene interactions in the structure and evolution of genetic systems. Nat Rev Genet. 2008;9:855.
- 38.
Shen R, Ma JF. Distribution and mobility of aluminium in an Al-accumulating plant, Fagopyrum esculentum Moench. J Exp Bot. 2001;52:1683–7.
- 39.
Shen R, Ma J, Kyo M, Iwashita T. Compartmentation of aluminium in leaves of an Al-accumulator, Fagopyrum esculentum Moench. Planta. 2002;215:394–8.
- 40.
Ma JF. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int Rev Cytol. 2007;264:225–52.
- 41.
Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol. 1998;116:155–63.
- 42.
Sivaguru M, Liu J, Kochian LV. Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. Plant J. 2013;76:297–307.
- 43.
Zheng SJ, Ma JF, Matsumoto H. High aluminum resistance in buckwheat I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol. 1998;117:745–51.
Acknowledgments
We thank S. K. Giri for technical assistance for ICP-MS analyses.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (41877423) to Y.W. and a USDA-ARS CRIS fund (1907–21000-034-00D) to J.L.
Author information
Affiliations
Contributions
J.L. conceived and supervised the research. Y.W. and J.L. designed the experiments. Y.W., W.Y., Y. Cao, Y. Cai, S.M.L., W.W., Y.K. and C.L. performed the experiments and analyzed the results. J.L. and Y.W. wrote the paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
春天er Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1 Figure S1.
Seeds ofWTandnip1;2–1, nip1;2–2andnip1;2–3mutants were germinated and grown in hydroponic solution (pH 4.3) supplemented with 20 μM of AlCl3for 5 days.Figure S2.Seeds ofWT, almt1, mate, nip1;2andalmt1_mate, almt1_nip1;2double mutants were germinated and grown in hydroponic solution (pH 4.3) supplemented without (−Al) or with (+Al) 20 μM of AlCl3for 5 days.Figure S3.Root growth of different genotypes of the F2 population.Figure S4.Gene structure, positions of T-DNA insertions and PCR primers ofALMT1andNIP1;2.Figure S5.Gene structure, position and size of real-time RT-PCR amplicon forALMT1,NIP1;2and18S rRNA.
Rights and permissions
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Yu, W., Cao, Y.et al.一个排斥机制是一个内部的上位detoxification mechanism in aluminum resistance in Arabidopsis.BMC Plant Biol20,122 (2020). https://doi.org/10.1186/s12870-020-02338-y
Received:
Accepted:
Published:
Keywords
- ALMT1
- Epistasis
- Malate
- NIP1;2
- Organic acid
- Resistance mechanism
- Root cell wall