- Research article
- Open Access
- Published:
Inhibition of multiple defense responsive pathways by CaWRKY70 transcription factor promotes susceptibility in chickpea underFusarium oxysporumstress condition
BMC Plant Biologyvolume20, Article number:319(2020)
Abstract
Background
Suppression and activation of plant defense genes is comprehensively regulated by WRKY family transcription factors. Chickpea, the non-model crop legume suffers from wilt caused byFusarium oxysporumf. sp.ciceriRace1 (Foc1), defense response mechanisms of which are poorly understood. Here, we attempted to show interaction between WRKY70 and several downstream signaling components involved in susceptibility/resistance response in chickpea upon challenge with Foc1.
Results
In the present study, we foundCicer arietinumL. WRKY70 (CaWRKY70) negatively governs multiple defense responsive pathways, including Systemic Acquired Resistance (SAR) activation in chickpea upon Foc1 infection. CaWRKY70 is found to be significantly accumulated at shoot tissues of susceptible (JG62) chickpea under Foc1 stress and salicylic acid (SA) application.CaWRKY70overexpression promotes susceptibility in resistant chickpea (WR315) plants to Foc1 infection. Transgenic plants upon Foc1 inoculation demonstrated suppression of not only endogenous SA concentrations but expression of genes involved in SA signaling.CaWRKY70overexpressing chickpea roots exhibited higher ion-leakage and Foc1 biomass accumulation compared to control transgenic (VC) plants. CaWRKY70 overexpression suppresses H2O2生产和合成活性氧(ROS) induced cell death in Foc1 infected chickpea roots, stem and leaves. Being the nuclear targeted protein, CaWRKY70 suppresses CaMPK9-CaWRKY40 signaling in chickpea through its direct and indirect negative regulatory activities. Protein-protein interaction study revealed CaWRKY70 and CaRPP2-like CC-NB-ARC-LRR protein suppresses hyper-immune signaling in chickpea. Together, our study provides novel insights into mechanisms of suppression of the multiple defense signaling components in chickpea by CaWRKY70 under Foc1 stress.
Conclusion
CaWRKY70 mediated defense suppression unveils networking between several immune signaling events negatively affecting downstream resistance mechanisms in chickpea under Foc1 stress.
Background
Plant defense against pathogens are rapidly conveyed through cell surface receptors or by the intracellular immune receptors. Cell surface receptors usually recognize specific pathogen or microbe associated molecular patterns (i.e., PAMPs or MAMPs) and elicits Pattern Triggered Immunity (PTI). By contrast, intracellular receptors bind PTI suppressing effector proteins released by the pathogens which induce strong immune response, known as Effector Triggered Immunity (ETI) [1,2]。WRKY transcription factors (TFs) are indispensable regulators of both PTI and ETI to wide variety of pathogens. The members of large multigene family transcription factor comprise WRKY domain (WRKYGQK) and zinc finger motif (CX4-7CX22-23HXH/C) that binds at TGAC core of W-box containing DNA [3,4]。There are 74 WRKY family members present inArabidopsis thaliana, which have been classified into three major groups (I, II and III) based on the number and position of WRKY domains and features of the zinc finger motif [5]。
Transcriptional regulation of plant defense related gene expression by WRKY proteins are crucial to enable the induction of host immunity. Binding of WRKY70 TF at promoters of SA and JA signaling pathway genes, such asNPR1,PR2,PR10,VSP1andVSP2are associated with positive regulation of plant defense signaling [6,7,8,9]。AtWRKY33overexpression leads to enhanced resistance against necrotrophic fungal pathogens,Botrytis cinereaandAlternaria brassicicola, although, plants showed susceptibility toPseudomonas syringaeinfection [10]。WRKY28 and WRKY46 play co-transcriptional regulators ofISOCHORISMATE SYNTHASE1(ICS1) gene expression and SA biosynthesis which mount defense response against biotrophic pathogens [11]。SA accumulation severely affects both PTI and ETI [12,13]。Activation of Systemic Acquired Resistance (SAR) in the pathogen free distal tissues is also dependent on SA accumulation and signaling that trigger resistance against a large variety of pathogens, including viruses, bacteria and fungi [14,15,16]。InArabidopsis,SA INDUCTION-DEFICIENT2(SID2),ENHANCED DISEASE SUSCEPTIBILITY5(EDS5), andNONEXPRESSOR OF PR GENES1(NPR1) control SA production and signaling on pathogen challenge [17]。SID2encodes an isochorismate synthase enzyme that converts chorismate to isochorismate [18]。Pathogen induced expression ofSID2,and concomitant SA accumulation is regulated bySYSTEMIC ACQUIRED RESISTANCE DEFICIENT 1(SARD1) [19]。SARD1 positively regulatesICS1gene expression that promotes pathogen-inducible SA accumulation inArabidopsis[19,20]。AtWRKY70 binds at promoter and inhibitsSARD1expression, which lowers the endogenous SA levels [20]。AtWRKY70 also functions as transcriptional regulator of JA/ ET induced gene expression and Induced Systemic Resistance (ISR) triggered byBacillus cereusAR156 [21]。The apparent positive or negative effects of AtWRKY70 on transcription may thus provide the mechanistic basis for regulation of SA induced defense gene expression during local and systemic resistance inArabidopsis.
ROS are the primary inducer for plant defense signaling that can trigger activation of mitogen activated protein kinase (MPK) cascade during plant-pathogen interplay [22,23]。ROS production leads to upregulation of genes involved in SA- and JA/ ET- signaling pathway [24]。Furthermore, SA and ROS together play crucial roles in hypersensitive response (HR) triggered cell death signaling during SAR development inArabidopsis[25]。Respiratory Burst Oxidase Homologs (RBOHs), a plasma membrane bound NADPH oxidase contribute ROS production inArabidopsis thalianaandNicotiana benthamiana[26,27]。WRKYs are the transcriptional regulator of ROS production in these plants. WRKYs regulate the expression ofAtRBOHDandAtRBOHFthat mediate ETI-induced ROS bursts [26]。WRKY8 triggersNbRBOHBexpression and HR induced cell death inN. benthamiana[27]。Treatment ofArabidopsisleaves with H2O2, a primary ROS candidate also upregulates the expression of manyWRKYgenes [28]。Thus,WRKYgenes expression and ROS production are coordinately regulated at transcriptional level that prompts the activation of multiple defense signaling pathways like, hormonal crosstalk, ROS signaling, MAPK signaling, and HR associated cell death.
HR develops only when an appropriate Avr (avirulent) protein interacts with its cognate R (resistance) proteinin planta[29,30]。Effector proteins often target WRKYs in order to manipulate plant immunity. It is a well-known fact that WRKYs and R proteins serve common regulators of resistance signaling pathways to several plant-pathogen interactions.ArabidopsisResistance toRalstonia solanacearum1 (RRS1) carries an extra integrated WRKY domain at its C-terminal end. This type of extended WRKY module perceives PopP2 effector protein and protects acetylation of other WRKYs upon instigating strong immune responses to the bacterial pathogenR. solanacearum[31]。It is important that RRS1 with its single WRKY domain can induce transcriptional reprogramming during ETI. WRKY70 also contributes to Recognition ofPeronospora Parasitica4 (RPP4)-mediated resistance againstHyaloperonospora parasitica[32]。Our recent study has established that Foc1 resistance in chickpea is dependent on the interaction between RPP2-like CC-NB-ARC-LRR protein and CaWRKY64 [30]。
The present study has been focussed on chickpea-Fusariuminteraction since, a smaller number of reports are currently available on legume-fungus interactions and detailed molecular regulations are undoubtedly obscured. Chickpea (Cicer arietinumL.) is the world’s third most important pulse crop and a rich source of plant-derived edible protein. Chickpea production has been severely affected by wilt-causing hemi-biotrophic fungusFusarium oxysporumf. sp.ciceriRace1 (Foc1) [33]。Amongst the eight different pathogenic races ofFusarium oxysporumf. sp.ciceri, Race1 is known to have cosmopolitan distribution causing significant yield losses. Foc1 infection accounting 10–15% annual crop loss and reaches 90–100% during favourable season [34,35]。Foc1 invades chickpea through roots and grows to shoots where it colonizes the xylem vessels at root-stem interface region. Increasing fungal biomass blocks water supply to the aerial shoots, which results in massive vascular wilting [34,35]。Foc1 resistance in chickpea is hard to achieve by usual breeding approaches due to limited genetic resources and elevated autogamy [36]。We used wilt-susceptible JG62 and wilt-resistant WR315 chickpea accessions to unveil the immunomodulatory role of CaWRKY70 protein on Foc1 infection [36,37]。Our study shows that CaWRKY70 transcription factor promotes susceptibility in chickpea upon Foc1 infection. CaWRKY70 inhibits SA concentrations and signaling in non-inoculated distal shoot tissues of transgenic chickpea. Transcripts measurement data suggests CaWRKY70 functions as negative regulator for subsets of immune-responsive genes that control defense responses in chickpea, includingPRgenes. CaWRKY70 also suppresses endogenous ROS levels and R-protein induced ectopic cell death. Together, we establish that CaWRKY70 negatively impacts defense signaling and SAR development in chickpea under Foc1 stress.
Results
Systemic expression pattern ofCicer arietinumL. WRKY70 (CaWRKY70) under SA induction and Foc1 infection
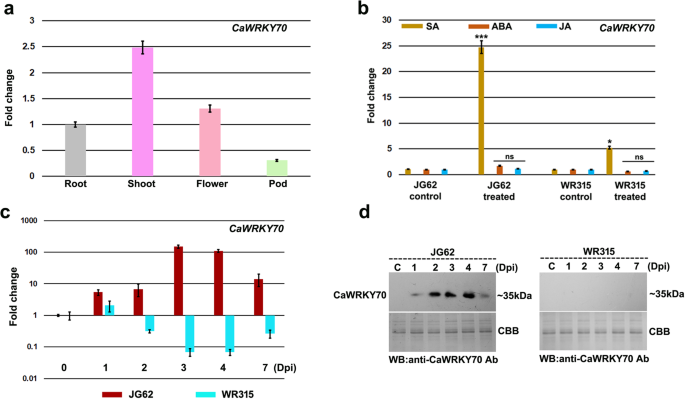
CaWRKY70expression in different chickpea tissues were determined by quantitative real-time PCR (qRT-PCR) analysis.CaWRKY70mRNA expression was detected in all tissues including root, shoot, flower and pod.CaWRKY70transcript accumulation was higher at shoot tissues compared to flower, root and pod in non-infected plants.CaWRKY70expression was detected to be ~ 2.5-fold higher at shoot tissues than the roots (Fig.1a). Differential salicylic acid (SA) accumulation induces SAR activation at distal shoot tissues of susceptible and resistant chickpea plants upon Foc1 infection [38]。To investigate whether SA signaling influencesCaWRKY70expression in chickpea, we measuredCaWRKY70transcript abundance in shoot tissues of susceptible and resistant chickpea at 6 h of SA treatment. Result suggests that SA treatment promotes significantly higher accumulation ofCaWRKY70transcript at shoot tissues of susceptible chickpea over control treatment. However, resistant plant shows less induction ofCaWRKY70transcript upon exogenous SA application (Fig.1b). Other inducers like, ABA and JA failed to stimulateCaWRKY70expression neither in susceptible nor in the resistant chickpea shoots. Therefore, it may be suggested that differential expression ofCaWRKY70in chickpea is mediated through SA response under Foc1 infection.ArabidopsisWRKY70 is an important WRKY member that has been shown to regulate SAR activation against biotrophic pathogens [9,39]。To ascertain whetherCaWRKY70is associated with the systemic defense responses of chickpea, we sought to determine its mRNA expression at shoot tissues under control treatment and Foc1 infection. Susceptible and resistant chickpea plants subjected to Foc1 infection at 1, 2, 3, 4 and 7 days were used for RNA isolation, cDNA preparation and qRT-PCR analyses.CaWRKY70expression at different time-points as compared to the 0 dpi control treatment in susceptible JG62 and resistant WR315 were plotted (Fig.1c).CaWRKY70fold change levels were normalized to a value of 1 at 0 dpi in JG62 and WR315, respectively.CaWRKY70transcript was found to be induced at shoot tissues of susceptible chickpea on challenge with Foc1. On the contrary, resistant chickpea plants were unable to stimulateCaWRKY70expression at shoot tissues under Foc1 stress. Time-dependent data revealed thatCaWRKY70transcript was found to be ~ 100-fold upregulated at 3 dpi in susceptible chickpea shoots over control treatment (Fig.1c). However, the inclusion of mock treatment to each time point would have been useful to determine the developmental stage specificCaWRKY70expression in both susceptible and resistant chickpea upon Foc1 infected conditions. To investigate CaWRKY70 protein accumulation, total proteins were extracted from control and Foc1 infected susceptible and resistant chickpea shoot tissues. Next, immunoblotting experiment was performed using anti-CaWRKY70 polyclonal antibody. Result shows induction of ~ 35 kDa CaWRKY70 protein band in non-inoculated systemic shoot tissues of susceptible plants upon Foc1 infection, whereas resistant plants failed to stimulate such protein accumulation (Fig.1d). The protein level was higher at shoot tissues of susceptible plant at 4 dpi on Foc1 challenge, suggesting systemic accumulation of CaWRKY70 in susceptible chickpea.
Expression pattern of CaWRKY70 transcript and protein in chickpea.aOrgan specific expression ofCaWRKY70in chickpea root, shoot, flower and pod tissues, respectively.CaWRKY70mRNA levels in different organs were measured usingCaGAPDHas internal control. Error bars represent mean ± SD of three independent biological replicates.bCaWRKY70transcript levels after SA, ABA and JA treatments in susceptible JG62 and resistant WR315 chickpea shoots.CaGAPDHwas used as internal control. Error bars represent ±SD (n = 3). Asterisks (*) indicate values that differ significantly from control treatment (0 dpi) as determined by Student’sttest (*P ≤ 0.05 and ***P ≤ 0.001). NS denotes not significant.cNormalized fold induction ofCaWRKY70transcript at shoot tissues of susceptible JG62 and resistant WR315 chickpea upon Foc1 infection.CaWRKY70fold change values at different time-points are normalized against 0 dpi in JG62 and those in WR315, respectively.CaGAPDHexpression was used as the reference control. Error bars represent ±SD values of three biological replicates.dCaWRKY70 protein accumulation was determined by western blotting with anti-CaWRKY70 antibody. Coomassie Brilliant Blue (CBB) stained gels serve loading control
CaWRKY70 is nuclear targeted protein
To test subcellular localization of CaWRKY70 protein, we fused yellow fluorescence protein (YFP) at its C-terminus. CaWRKY70 protein fused to YFP was expressed in onion epidermal cells byAgrobacterium介导的瞬态转变。相反,一个empty construct expressing control YFP was tested. Onion epidermal cells were stained with nuclear marker 4′, 6-diamidino-2-phenylindole (DAPI). Confocal microscopic analyses showed that CaWRKY70-YFP was localized in nucleus. In contrast, localization of control YFP protein was observed throughout the cells (Additional file1: Figure S1). Result also revealed that blue color fluorescence of DAPI-stained nuclei is overlapped with yellow color fluorescence of YFP, thereby, confirming nuclear localization of CaWRKY70.
CaWRKY70overexpression triggers susceptibility in chickpea to Foc1
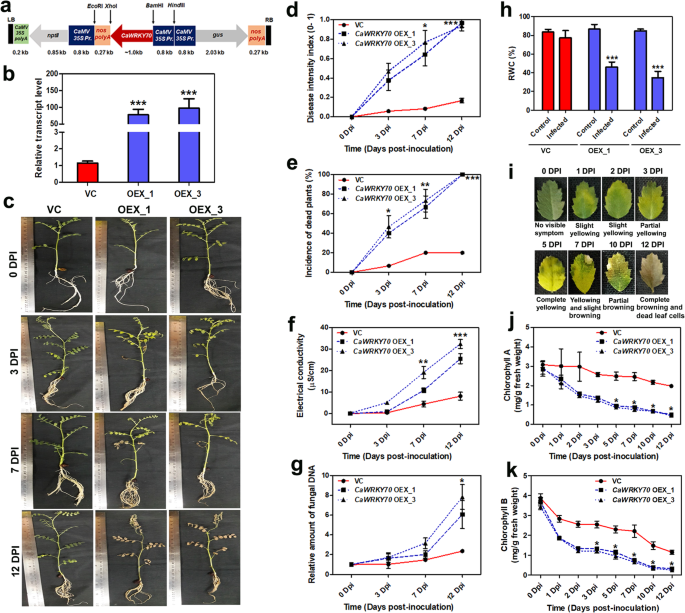
To examine the possible role ofCaWRKY70in defense regulation against Foc1, full-lengthCaWRKY70gene was isolated from Foc1 treated shoot tissues of susceptible chickpea cDNA sample. ChimericCaWRKY70gene construct was prepared using pCAMBIA2301 vector and positive clones were selected by PCR analyses (Additional file1: Figure S2).CaWRKY70gene containing plasmids were delivered into resistant chickpea genome viaAgrobacteriummediated transformation and transgenic chickpea plants were established (Fig.2a, b). Two independentCaWRKY70overexpressing chickpea lines i.e., OEX1, and OEX3 of T2generation were obtained from primary transformants. Real-time PCR analysis detected that overexpressing plants exhibit significantly higher expression ofCaWRKY70transcript than control vector transformed plants, where lowest expression ofCaWRKY70mRNA was noted (Fig.2b). To compare disease symptoms on control transgenic andCaWRKY70overexpressing chickpea, we treated plants with Foc1 for 0, 3, 7 and 12 days, respectively. Result shows thatCaWRKY70overexpressing chickpea plants exhibit enhanced susceptibility to Foc1 infection in comparison to the control transgenic plants. Disease symptoms were much pronounced in overexpressing chickpea plants at 12 dpi with Foc1 (Fig.2c). Foliar symptom that developed on control transgenic andCaWRKY70overexpressing chickpea upon interaction with Foc1 was used to measure the disease intensity index. Based on incidence of infected plants and foliar symptoms,CaWRKY70overexpressing chickpea was found to be highly susceptible to Foc1 than control transgenics. Disease symptom started developing at 3 dpi, progressed at higher rates inCaWRKY70overexpressing鹰嘴豆导致100%植物with vascular wilt developed at 12 dpi (Fig.2d).CaWRKY70transgenic plants developed highly susceptible reaction to Foc1 reaching 100% incidence of dead plants at 12 dpi. In contrast, control transgenic plants demonstrated less severe reaction at 12 dpi with only 20% of the plant’s dead (Fig.2e). Foc1 infection progressively enhances cell-death induced ion-leakage at root tissues of control transgenic andCaWRKY70overexpressing chickpea. Time-dependent analyses show significant difference in Foc1 induced electrolyte leakage in control transgenic andCaWRKY70overexpressing chickpea with higher leakage of ions in overexpressing chickpea roots and least in control transgenic plants. The conductivity was found to be significantly increased inCaWRKY70overexpressing root at 12 dpi of Foc1 infection than control transgenics (Fig.2f). Amount of Foc1 biomass in root tissues ofCaWRKY70overexpressing chickpea appears to be significantly higher than control transgenic. The relative accumulation of Foc1 5.8S rDNA was ~ 2.0-fold and ~ 7.0-fold at root tissues of control transgenic andCaWRKY70overexpressing chickpea, respectively, under Foc1 infection (Fig.2g). Relative water content (RWC) was determined to compare percentage amount of water restored within the plant body of control transgenic andCaWRKY70overexpressing chickpea under control condition and Foc1 infection. Result shows that control transgenic plants retain ~ 77% RWC upon Foc1 infection whereas, the value was markedly reduced inCaWRKY70overexpressing chickpea plants under Foc1 stress i.e., ~ 46% and ~ 34% RWC (Fig.2h). Foc1 inoculated chickpea plants show chlorosis of leaves accompanied by yellowing to browning or cell death (Fig.2i). Decrease in chlorophyll A and chlorophyll B content was measured using leaves of control transgenic andCaWRKY70overexpressing chickpea in time dependent manner. Control transgenic plants retain significantly higher chlorophyll A and chlorophyll B content than overexpressing chickpea on Foc1 stress (Fig.2j, k). Nevertheless, loss of total chlorophyll content in chickpea leaves might be a secondary effect of Foc1 infection. Taken together, our results confirm thatCaWRKY70promotes susceptibility in chickpea to Foc1.
CaWRKY70overexpression induces susceptibility in chickpea under Foc1 infection.aDiagram of gene construct used forCaWRKY70overexpression in resistant chickpea.bqRT-PCR determination ofCaWRKY70mRNA level in control transgenic (VC) and overexpressing chickpea. Error bars indicate ±SD of three independent biological samples. Data was normalized toCaGAPDH. Fold change values relative to control vector transformed plants.cDevelopment of disease symptom in control transgenic andCaWRKY70overexpressing chickpea. Disease phenotype of transgenic plants were photographed under control treatment (0 dpi) and Foc1infection at 3, 7 and 12 dpi, respectively.dAssessment of disease intensity index in control transgenic andCaWRKY70overexpressing chickpea under control treatment (0 dpi) and Foc1infection. Chickpea plants grown in the soil-rite mixture were inoculated with Foc1.eEffect of Foc1 infection on the control transgenic andCaWRKY70overexpressing chickpea based on incidence of dead or wilted plants. Indande, each data point represents mean values of three pots with five plants per pot. Error bars indicate ±SD of three independent biological samples.fElectrolyte leakage in control transgenic (VC) andCaWRKY70overexpressing chickpea roots under control treatment (0 dpi) and Foc1 infection. Each time point represents ±SD of three biological replicates. *P ≤ 0.05 and **P ≤ 0.01 indicate values show significant differences between control transgenic (VC) andCaWRKY70overexpressing chickpea as determined by Student’sttest.gQuantitation of Foc1 5.8S rDNA at root tissues of control transgenic (VC) andCaWRKY70overexpressing chickpea plants by real-time PCR. Chickpea DNA amount was normalized byCaGAPDH表达式。每个栏代表的意思是±SD的三个independent biological replicates and fold change is relative to control treatment (0 dpi). **P ≤ 0.01 indicate mean values significantly different from control vector transformed plant determined by Student’sttest.hRelative water content percent (RWC%) in control transgenic andCaWRKY70overexpressing chickpea under control treatment and Foc1 infection. Asterisks (*) indicate significant difference at ***P ≤ 0.001 by one-way ANOVA followed by multiple comparison of means using tukey’s post-hoc test.iDisease symptoms in chickpea leaves for Foc1 varied from yellowing to browning or cell death at different infection time-points.jChlorophyll A and (k) Chlorophyll B content in control transgenic andCaWRKY70overexpressing chickpea leaves at different infection time-points. Injandk, error bars represent ±SD (n = 3). *P ≤ 0.05 indicate mean values are significantly different from control vector transformed plants as determined by Student’sttest
CaWRKY70 reduces ROS accumulation and cell death in chickpea
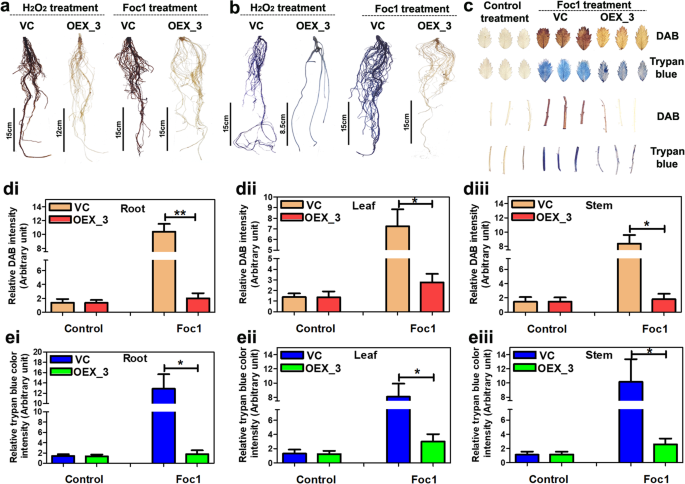
Histochemical DAB and trypan blue staining was performed to compare H2O2accumulation and cell death between control transgenic andCaWRKY70overexpressing chickpea under Foc1 stress. Result demonstrates intense DAB and trypan blue colouration in Foc1 infected control transgenic roots (Fig.3a, b). On the contrary, DAB and trypan blue staining was not observed atCaWRKY70overexpressing chickpea roots under Foc1 stress. H2O2treatment was carried out as primary ROS inducer in transgenic chickpea roots which show intense DAB and trypan blue staining at roots of control transgenic plants. DAB colouration was not demonstrated byCaWRKY70overexpressing chickpea roots. However, faint trypan blue colour was retained by overexpressing plant roots only after H2O2treatment (Fig.3a, b). DAB and trypan blue staining was also performed using leaves and stem parts of the control transgenic andCaWRKY70overexpressing chickpea plants. Here, no such DAB or trypan blue staining was noted upon control treatment, however, Foc1 infection resulted in higher deposition of brownish DAB precipitates in leaves and stem tissues of control transgenic plants in comparison toCaWRKY70overexpressing chickpea (Fig.3c). Similarly, strong trypan blue colouration was noted on leaves and stem portions of control transgenic plants thanCaWRKY70overexpressing chickpea upon Foc1 infection (Fig.3c). It is noteworthy thatCaWRKY70overexpressing chickpea leaves and stem show mild histochemical DAB and trypan blue staining. We further measured DAB and trypan blue colour intensities of control transgenic andCaWRKY70overexpressing chickpea root, leaf and stem tissues. Quantitative measurements revealed significant reduction in the DAB and trypan blue intensities at roots, leaves and stem parts ofCaWRKY70overexpressing chickpea compared to control transgenic plants under Foc1 stress (Fig.3d, e). Although, control treatment does not exhibit such drastic changes in DAB and trypan blue intensities of empty vector transformed andCaWRKY70overexpressing chickpea plants. Therefore, it can be concluded that CaWRKY70 negatively affects ROS accumulation and cell death induction and thus promotes susceptibility inCaWRKY70overexpressing chickpea plants upon Foc1 infection.
H2O2accumulation and cell death in different chickpea organs of control transgenic andCaWRKY70overexpressing plants upon Foc1 infection.a,bDAB and trypan blue staining of chickpea roots under H2O2treatment and Foc1 infection.cDAB and trypan blue colouration of leaves and stem portions from control transgenic andCaWRKY70overexpressing chickpea plants. Ina,bandc, chickpea plants were infected with Foc1 for 7 days. 10 mM H2O2treatment was carried out for 30 min using chickpea roots.dQuantification of relative DAB staining activities in control transgenic andCaWRKY70overexpressing chickpea roots (i), leaves (ii) and stem (iii) under control treatment Foc1 infection. (e) Quantitation of relative trypan blue colour intensities in control transgenic andCaWRKY70overexpressing chickpea roots (i), leaves (ii) and stem (iii) under control treatment (0 dpi) and Foc1 stress. Indande, error bars represent ±SD (n = 3). Asterisks (*) indicate values which are significantly different between control transgenic andCaWRKY70overexpressing chickpea plants as determined by Student’sttest (*P ≤ 0.05 and **P ≤ 0.01)
CaWRKY70 inhibits SA signaling in transgenic chickpea
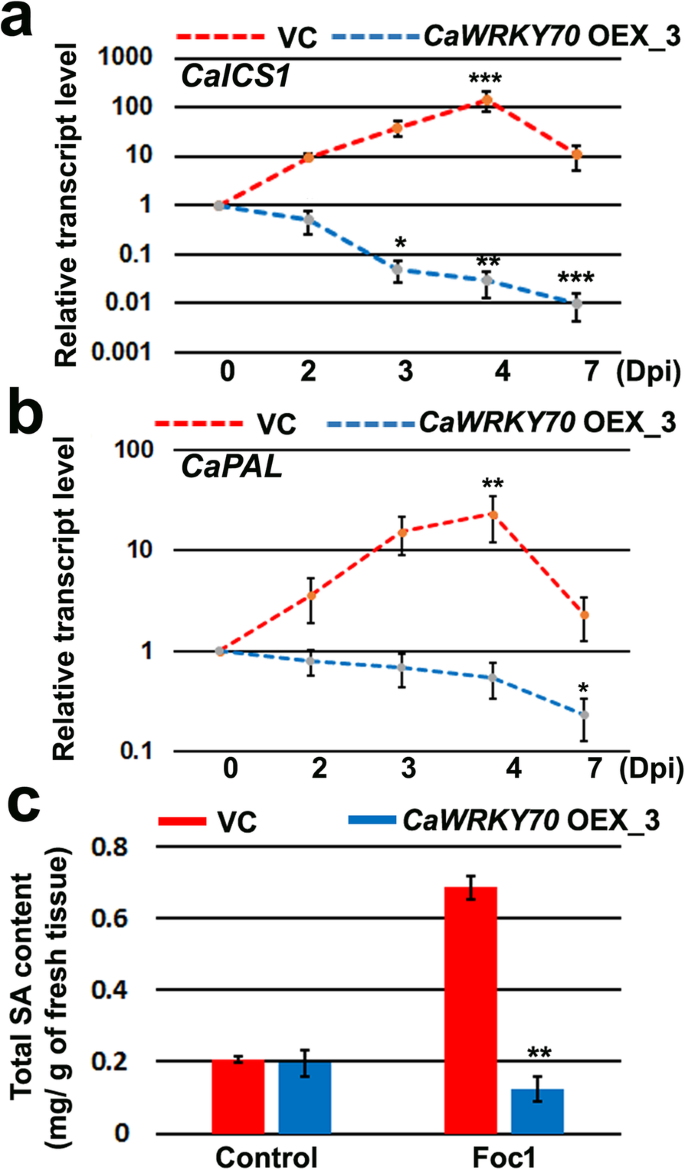
ICS1 and PAL contribute to the pathogen induced SA production through isochorismate and phenyl-propanoid pathways, respectively [18,40]。InCaWRKY70overexpressing chickpea shoots,CaICS1andCaPALtranscripts were found to be downregulated after Foc1 infection at 2, 3, 4 and 7 days. Interestingly, pCAMBIA2301 vector transformed plant demonstrates mRNA induction upto 4 dpi and then downregulation at 7 dpi under Foc1 stress (Fig.4a, b). SA concentrations were also significantly decreased at shoot tissues ofCaWRKY70overexpressing chickpea in comparison to the control transgenic plants at 7 dpi with Foc1 (Fig.4c). Result shows higher accumulation of SA in non-inoculated systemic shoot tissues of control vector transformed chickpea after Foc1 infection. It has been observed that control transgenic andCaWRKY70overexpressing chickpea exhibit basal accumulated level of SA production at shoot tissues under control treatment. Thus, CaWRKY70 reduces both SA biosynthesis genes expression and SA accumulation at shoot tissues of transgenic chickpea.
SA signaling gene expression and SA concentrations at shoot tissues transgenic chickpea under Foc1 stress.aCaICS1(XM_004514070.3), (b)CaPAL(NM_001279177.2) transcripts level at shoot tissues of control transgenic (VC) andCaWRKY70overexpressing chickpea under Foc1 infection.CaGAPDHwas used as internal control. Inaandb, Error bars represent mean values ±SD (n = 3). Asterisks (*) indicate values that differ significantly from control treatment (0 dpi) as determined by Student’sttest (*P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001). (c) Total SA content at control transgenic (VC) andCaWRKY70overexpressing chickpea shoot tissues under control treatment (0 dpi) and Foc1 infected condition (7 dpi). Each bar represents mean ± SD of three independent biological samples. **P ≤ 0.01 indicates mean values showing significant difference between control transgenic andCaWRKY70overexpressing chickpea by Student’sttest
Differential expression pattern of defense related transcripts in vector transgenic andCaWRKY70overexpressing chickpea under Foc1 infection
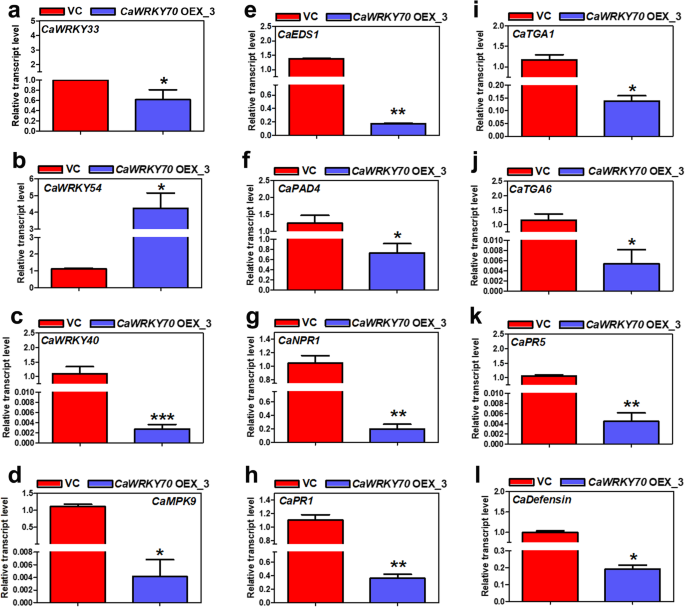
We compared the expression of defense responsive transcripts between vector inoculated andCaWRKY70overexpressing transgenic chickpea on Foc1 challenge.CaWRKY33transcript was remarkably lower at the shoot tissues of overexpressing chickpea than vehicle transgenics (Fig.5a). WRKY54 and WRKY70 are the defense related transcription factors that negatively regulate osmotic stress tolerance inArabidopsis[41]。At shoot tissues ofCaWRKY70overexpressing chickpea,CaWRKY54mRNA was upregulated after Foc1 treatment (Fig.5b).CaWRKY40expression inArabidopsisenhanced resistance to virulentPseudomonas syringaepv. tomato DC3000 infection [42]。Furthermore, CaMPK9-CaWRKY40 signaling promotes primary defense responses in chickpea against Foc1 [43]。CaWRKY70inhibitsCaWRKY40expression in transgenic chickpea (Fig.5c).CaWRKY70overexpressing chickpea also demonstrates a sharp decrease inCaMPK9transcript level (Fig.5d). EDS1 and PAD4 complex formation was found to be required for pathogen infection induced SA accumulation [44]。However, bothCaEDS1andCaPAD4mRNAs were downregulated inCaWRKY70overexpressing chickpea plant type (Fig.5e, f). Recent finding showed that phosphorylation dependent changes in AtNPR1 promotes its interaction with AtWRKY70 which suppressesPathogenesis Related(PR) gene transcription inArabidopsis[45]。Present result suggests thatCaNPR1,CaPR1andCaPR5transcript levels were reduced inCaWRKY70overexpressing chickpea in comparison to the vehicle treated plants (Fig.5g, h and k). Among other SA signaling genes, expression ofCaTGA1andCaTGA6mRNAs were downcast inCaWRKY70overexpressing chickpea (Fig.5i, j). Induction of JA-signaling geneCaDefensinmRNA is also inhibited at shoot tissues ofCaWRKY70over-accumulating植物比反式控制向量formed chickpea (Fig.5l). Overall, CaWRKY70 negatively regulates induction of defense related genes expression at shoot tissues of transgenic chickpea upon Foc1 infection.
Defense related genes expression in transgenic chickpea on Foc1 stress.aCaWRKY33(XM_004490620.3), (b)CaWRKY54(based onGlycine max WRKY54DQ322698.1), (c)CaWRKY40(XM_004507020.3), (d)CaMPK9(XM_004505883.3), (e)CaEDS1(XM_004506171.2), (f)CaPAD4(XM_012716750.1), (g)CaNPR1(XM_012716326.2), (h)CaPR1(XM_004487759.2), (i)CaTGA1(XM_027334567.1), (j)CaTGA6(XM_012715238.2), (k)CaPR5(AJ487040.1), (l)CaDefensin(DQ288897.2)。数据提出了均值±SD (n =3). Asterisks (*) indicate values are significantly different from control transgenic (VC) plants as measured by Student’sttest (*P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001)
CaWRKY70 repressesCaWRKY40promoter activity
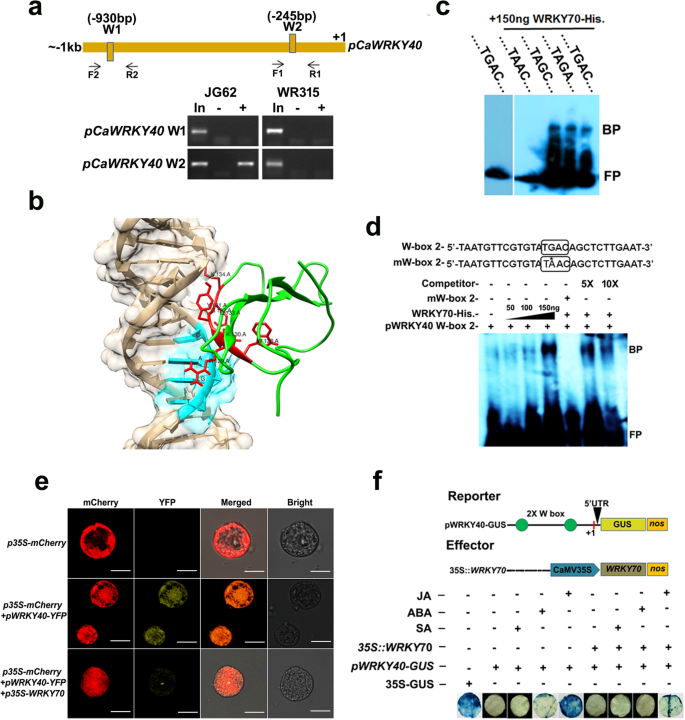
As described above,CaWRKY70overexpressing chickpea significantly lowered the expression ofCaWRKY40mRNA under Foc1 infection; we got interested to check whether CaWRKY70 plays any direct modulatory role in the suppression ofCaWRKY40promoter activity. ChIP-PCR assay revealed that CaWRKY70 physically associates with W-box 2 atCaWRKY40启动子。CaWRKY70 binding was observed at shoot tissues of susceptible (JG62) chickpea plants under Foc1 stress, whereas resistant chickpea shoot does not exhibit in vivo CaWRKY70 binding (Fig.6a). Next, we tested this binding through DNA-protein docking experiment. Phyre2 server used WRKY transcription factor 1 (PDB ID: c2aydA) as template structure with 100% confidence and 34% coverage (Additional file1: Figure S3a-c). Furthermore, qualitative assessment of the predicted model was evaluated by Ramachandran plot analysis using RAMPAGE server. Ramachandran plot analyses revealed that in CaWRKY70 model 94.7% residues are in favoured region, 5.3% residues are in the allowed region and none of the residues in outlier location (Additional file1: Figure S3d-e). For understanding the molecular mechanism of its interaction with W-Box DNA, an in silico DNA-protein docking was carried out with HADDOCK (Fig.6b). HADDOCK web server clustered 174 structures in 12 clusters, representing 87.0% of the HADDOCK generated water-refined models. Clusters were ranked according to HADDOCK score and Z-score for plotting DNA-protein interaction. Top five best scoring clusters are provided in Additional file2: Table S1. HADDOCK score is calculated as the weighted sum of van der Waals, electrostatic, desolvation and restraint violation energies whereas, the Z-score indicates how many standard deviations from the average of these clusters is in terms of score. For predicted protein model, HADDOCK score v/s i-RMSD (interface-RMSD) plot was created. i-RMSD was calculated based on the backbone (CA, C, N, O, P) atoms of all residues involved in intermolecular contact using 10 Å cut-off. l-RMSD (ligand-RMSD) was also calculated on the backbone atoms of all (N > 1) molecules (Additional file2: Table S1, Fig.6b). DNA-protein interaction was further confirmed by in vitro EMSA. Result indicates in vitro CaWRKY70 binding to wild-type (TGAC), and mutated W-boxes (TAGC and TAGA) (Fig.6c). Approximately, 150 ng of purified histidine tagged CaWRKY70 exhibits strong DNA binding to W-box 2 atCaWRKY40启动子。However, such binding was completely inhibited upon mutating G nucleotide of TGAC i.e., TAAC. In vitro DNA binding of CaWRKY70 was outcompeted using 5 and 10 M excess cold probes, respectively (Fig.6d). Effect of CaWRKY70 binding at W-box 2 ofCaWRKY40promoter was tested by transient co-infiltration experiments usingNicotiana xanthiprotoplasts andNicotiana tabacumleaf discs, respectively. Protoplast co-transfection experiment showed that CaWRKY70 effectively inhibitsCaWRKY40promoter mediated expression of YFP inN. xanthiprotoplast, which suggests CaWRKY70 mediated negative regulation ofCaWRKY40promoter activity in vivo (Fig.6e).Agrobacterium-mediated transient co-infiltration ofp35S:CaWRKY70(effector construct) andpCaWRKY40:GUS(reporter construct) in tobacco leaf discs demonstrates reduction inCaWRKY40promoter driven GUS expression (Fig.6f). Quantitative data also revealed ~ 2.5-fold reduction in the histochemical GUS staining upon constitutive induction of CaWRKY70 (Additional file1: Figure S4). Therefore, CaWRKY70 binds to and repressesCaWRKY40promoter activity.
CaWRKY70 suppressesCaWRKY40promoter activity in vivo andin planta.a体内染色质免疫沉淀反应(芯片)PCRssay shows CaWRKY70 binding to W-box 2 ofCaWRKY40promoter at shoot tissues of susceptible (JG62) chickpea under Foc1 stress. Diagram shows presence of W-boxes atCaWRKY40启动子。(在)表示从pre-cl输入放大eared chromatin samples. Arrow indicates position of the primers. Plus (+) and minus (−) signs indicate anti-CaWRKY70 antibody and pre-immune sera immunoprecipitated chromatins. (+ 1) denotes transcription start site (TSS).bIn silico molecular docking of CaWRKY70 and W-box 2 containingCaWRKY40promoter DNA of cluster 3.c, dElectrophoretic mobility shift assay (EMSA) shows in vitro histidine tagged WRKY70 binding atpWRKY40W-box 2. Approximately, 200 ng of WRKY70-His protein specifically binds at W-box 2 (− 217 to − 245 bp upstream of TSS). Plus (+) and minus (−) signs indicate presence or absence of specific components. BP indicates bound probe and FP represents free probe. Box indicates W-box 2. Asterisk (*) indicates mutated W-box. The experiment was repeated twice with similar results.eCaWRKY70 mediated trans-inhibition ofCaWRKY40promoter activity.p35S:CaWRKY70andpWRKY40:YFPconstructs were co-transfected in protoplasts obtained fromNicotiana tabacumcv. Xanthi. (Brad) cell suspension culture. mCherry was used as transformation marker. Scale bar = 10 μm. (f) CaWRKY70 reduces GUS expression driven byCaWRKY40promoter in tobacco leaf discs. Diagram shows constructs used forAgrobacteriummediated transient co-infiltration experiment. Plus (+) and minus (−) signs indicate presence or absence of specific vehicles
CaWRKY40 positively regulatesCaMPK9promoter activity
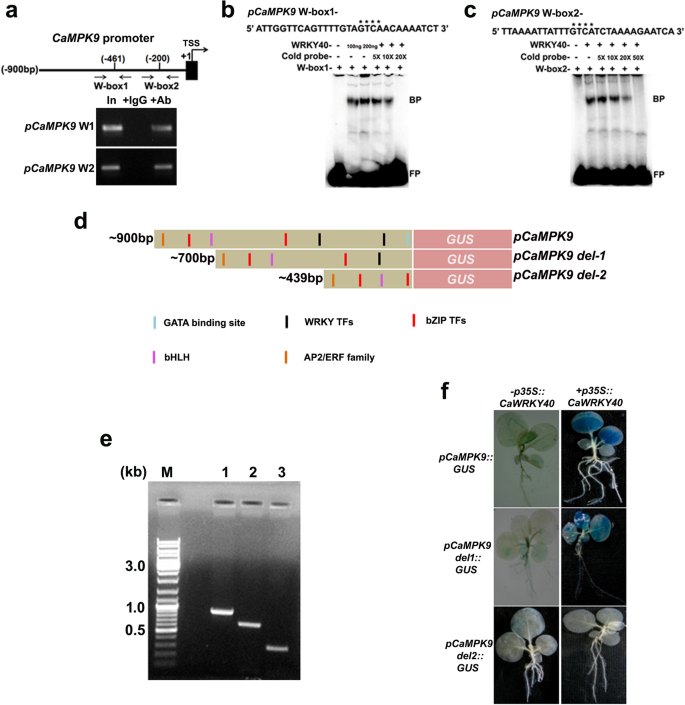
Recent report shows that higher activation of CaMPK9 in resistant chickpea phosphorylates CaWRKY40 under Foc1 stress [43]。We further anticipated that phosphorylated CaWRKY40 positively regulatesCaMPK9expression via feed-back mechanism in resistant chickpea on Foc1 challenge. Here, increased expression ofCaMPK9transcript in resistant chickpea also suggests its positive regulatory role in the defense activation against Foc1. Transcript level was found to be ~ 3.0-fold higher in resistant chickpea plants over control treatment. By contrast, susceptible chickpea plants show sharp downregulation ofCaMPK9transcript upon Foc1 challenge (Additional file1: Figure S5). ChIP-PCR data supports in vivo association of CaWRKY40 with W-boxes atCaMPK9promoter in resistant genotype plants upon exposed to Foc1 (Fig.7a). EMSA was used to establish in vitro binding of recombinant WRKY40 protein atCaMPK9promoter DNA. Binding reactions were performed using 200 ng purified 6 × histidine-tagged WRKY40 protein. Result shows the formation of sharp bound complexes after incubation of recombinant WRKY40 protein and labelled W-boxes. The complexes were competed out using 20 (for W-box 1) or 50 (for W-box 2) molar excess cold competitors (Fig.7b, c). To ascertain the specific role of CaWRKY40 inCaMPK9promoter activation, W-box-specific deletion constructs were generated and stably transformed into tobacco genome byAgrobacteriummediated gene transfer method (Fig.7d). Integration of theCaMPK9promoter fragments within tobacco genome was further confirmed by genomic PCR. Result shows sharp amplification ofCaMPK9promoter deletion derivatives (Fig.7e).CaMPK9promoter activity was further monitored in the presence or absence of specific effector constructs that constitutively express CaWRKY40. Reduction in the GUS activity was highest when both W-boxes were deleted (Fig.7f). However, deletion of a single W-box results in mild reduction of the GUS activity in effector construct-infiltrated setup. These results suggest that CaWRKY40 binds atCaMPK9promoter via both W-box 1 and W-box 2, which in turn positively modulatesCaMPK9表达式。
CaWRKY40 upregulates the activity ofCaMPK9promoterin planta.aIn vivo binding of CaWRKY40 toCaMPK9promoter at W-boxes. Schematic representsCaMPK9promoter and the W-boxes relative to transcription start site (+ 1). Arrows indicate the sites for primer binding.In plantaimmunoprecipitation of WRKY40-chromatin complex from susceptible and resistant chickpea shoots with anti-CaWRKY40 antibodies. Bound chromatins were eluted and used for PCR reactions. Sheared pre-cleared chromatin served as input control. Rabbit IgG was used as negative control for ChIP PCR.b, cIn vitro binding of recombinant WRKY40 to W-boxes atCaMPK9启动子。Approximately, 200 ng of His-WRKY40 protein was added to W-box containing labeledCaMPK9promoter fragment in an independent EMSA reaction. BP and FP indicate bound and free probes, respectively. Plus (+) and minus (−) signs denote presence or absence of individual elements. Asterisks highlight the W-boxes. Experiments were repeated twice with similar results.dDiagrams show 3’deletion fragments ofCaMPK9promoter and presence of the cis regulatory elements.eGenomic amplification ofCaMPK9promoter derivatives in transgenic tobacco by PCR.fTransactivation ofpCaMPK9:GUSin transgenicN. tabacumseedlings upon transient expression ofp35S:CaWRKY40
Ph值ysical interaction between CaWRKY70 and CC-NB-ARC-LRR protein suppresses cell death in chickpea
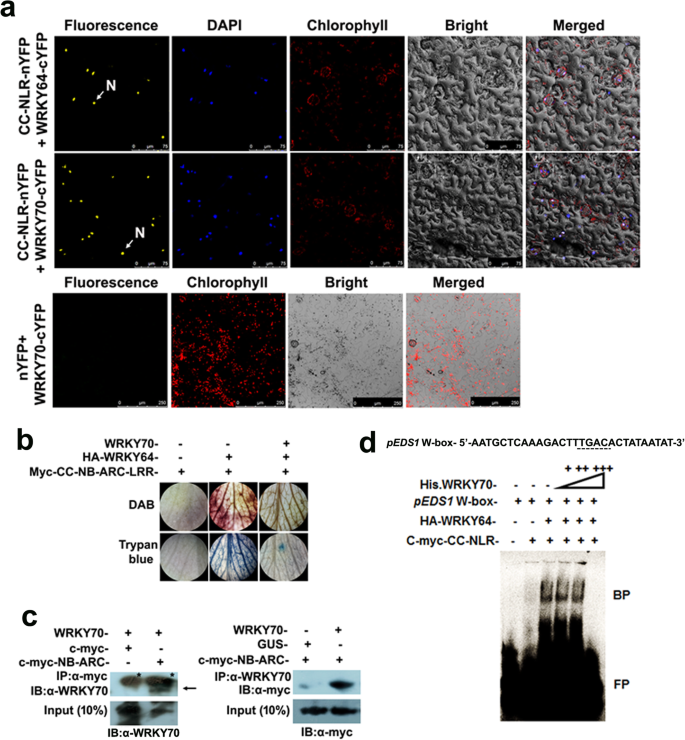
The present group has recently established that physical interaction between RPP2-like CC-NB-ARC-LRR (CC-NLR) protein and CaWRKY64 triggersin plantaectopic cell death [30]。To further investigate whether CaWRKY70 similarly influences cell death signaling by CC-NLR protein, we tested theirin plantainteraction through bimolecular fluorescence complementation (BiFC) assay usingNicotiana benthamianaleaves. Results demonstrate that CaWRKY64 and CaWRKY70 tagged to C-terminus of YFP (cYFP) interact with full-length CC-NLR protein fused to N-terminus of YFP (nYFP). Result shows that reconstitution of YFP signal was observed in the nucleus (Fig.8a). However, no such interaction was detected with control vector i.e., WRKY70-cYFP+nYFP. After testing their potential interaction between CaWRKY70 and CC-NLR protein, we were curious to check the effect of CaWRKY70 interaction on CaWRKY64 and CC-NLR protein mediated cell death phenomenon. Thus, we co-expressed HA tagged CaWRKY64, CaWRKY70 and myc tagged CC-NLR protein in chickpea leaves byAgrobacterium. The infiltrated leaves were further subjected to histochemical DAB and trypan blue staining. DAB staining shows that co-expression of epitope tagged CaWRKY64 and CC-NLR protein results in high levels of H2O2accumulation in infiltrated chickpea leaves, which is suppressed upon CaWRKY70 expression (Fig.8b). Similarly, trypan blue staining also depicted that CaWRKY70 effectively inhibits the retention of blue colouration and cell death in chickpea leaves when myc tagged CC-NLR protein and HA tagged CaWRKY64 were co-expressed (Fig.8b). Next, we performed co-immunoprecipitation (Co-IP) to show physical interaction between myc epitope tagged NB-ARC domain of CC-NLR protein and CaWRKY70. Proteins were transiently co-expressed inN. benthamianaleaves byAgrobacterium. Reciprocal Co-IP analyses detected that CaWRKY70 and myc-NB-ARC proteins were co-precipitated by anti-myc and anti-WRKY70 antibodies, respectively. Immunoblotting was performed with anti-WRKY70 and anti-myc antibodies. Similarly, input samples show the presence of both CaWRKY70 and myc-CC-NLR proteins after probed with anti-WRKY70 and anti-myc antibodies (Fig.8c). Effect of CaWRKY70 interaction on CC-NLR and CaWRKY64 mediated DNA binding has been further tested by in vitro EMSA experiment. Our group has previously shown that CC-NLR protein stimulates in vitro DNA binding of epitope tagged CaWRKY64 protein atCaEDS1promoter [30]。This binding was found to be reduced by the addition of an increasing amount of recombinant his-tagged WRKY70 protein (Fig.8d). Together, we establish that physical interaction between CaWRKY70 and CC-NLR protein negatively regulates cell death signaling in chickpea.
CaWRKY70 attenuates R-protein signaling in chickpea.aBiFC assay for physical interaction between CC-NLR-nYFP and CaWRKY70-cYFP. (nYFP + CaWRKY70-cYFP) and (CC-NLR-nYFP + CaWRKY64-cYFP) interactions were used as negative and positive controls, respectively. N denotes nucleus.bH2O2accumulation and cell death in chickpea leaves. Myc-CC-NLR, HA-WRKY64 and CaWRKY70 were transiently co-expressed in chickpea leaves byAgrobacterium. Infiltrated chickpea leaves were subjected to DAB and trypan blue staining.cCo-immunoprecipitation of c-myc-NB-ARC and CaWRKY70 afterAgrobacteriummediated transient expression inN. benthamianaleaves. Arrow indicates the immunoprecipitated protein band of CaWRKY70. Asterisks show non-specific bindings.dHis.-WRKY70 binding at W-box of chickpeaEDS1promoter by EMSA. Dotted lines represent W-box. BP and FP indicate bound and free probes, respectively. Inb, candd, plus (+) and minus (−) signs indicate presence or absence of specific components
Discussion
WRKY70 transcription factor is well characterized as an important transcriptional modulator of SA mediated signal transduction pathways inArabidopsisand wheat [39,46]。AtWRKY70overexpressingArabidopsisplants demonstrated enhanced resistance to biotrophic pathogensPseudomonas syringaeandErysiphe chichoracearum[39]。However, the plants showed hyper-susceptibility to necrotrophic fungusAlternaria brassicicola[9]。Contrastingly,Atwrky70mutants displayed susceptibility towardsB. cinereainfection [47]。In wheat,TaWRKY70positively regulates defense against stripe rust pathogenPuccinia striiformisf. sp.tritici[46]。BothArabidopsisand chickpea WRKY70 are group III members have been found to be positively involved in defense reaction to bacterial pathogenP. syringae[3,42,48]。Weak interaction of AtWRKY46 to AtWRKY70 and AtWRKY53 positively regulate the basal defense responses inArabidopsis[48]。AtWRKY46, AtWRKY70, and AtWRKY53 notably suppress JA-induced defense genes expression. Thus, involvement of WRKY70 protein in plant defense events is dynamic and host-pathogen specific. Despite its contribution in plant defense related functions, present group has already established its direct negative regulatory effects on abiotic stress responses in chickpea [49]。Here, we decipher immune suppressive functions of CaWRKY70 in chickpea shoots upon Foc1 infection.
SA response is common for induction of severalWRKYgenes involved in distinct stages of the SAR activation in plants [50,51]。SA treatment appears to be a positive inducer of SAR development andCaWRKY70expression in chickpea. Its mRNA level increases approximately 5-fold after 6 h of SA application over control treatment in susceptible plant compared to the resistant one (Fig.1).Likewise,AtWRKY70expression was also found to be induced almost 30-fold at 2 h post- SA treatment [9]。SA signaling invokesAtWRKY70expression in young and senescing leaves. Our result shows thatCaWRKY70expression was highest at shoot tissues of chickpea (Fig.1).Constitutive expression of bacterial salicylate hydroxylaseNahGremoves free SA and the subtle induction ofAtWRKY70transcript [52]。Arabidopsismutantseds1,pad4andnpr1compromised in SA signaling demonstrate reduced level ofAtWRKY70transcript, whereas mutant plantsedr1,cpr5andacd11exhibited SA hyperaccumulation and subsequent induction ofAtWRKY70[53,54]。SA and JA are two antagonistic signaling molecules that influence plant defense [55]。However, JA treatment was found to be ineffective forCaWRKY70transcript induction. In most cases, WRKY70 promotes SA-responsive genes expression and inhibits subset of JA-responsive genes [9]。Enhanced expression ofAtWRKY70incoi1mutant suggests that JA-responsive factor repressesAtWRKY70expression depending on endogenous JA levels [9]。Although, CaWRKY70 suppresses the expression of SA biosynthesis and signaling genes at shoot tissues of transgenic chickpea upon Foc1 infection (Figs.4and5).ICS1andPALexpression positively influences activation of SA signaling pathways and phenyl propanoid biosynthesis pathways leading to the production of anti-microbial secondary metabolites that protects chickpea and tomato plants from nematode penetration andFusarium oxysporuminfection, respectively [56,57]。Our previous transcriptomic and metabolite analyses also revealed induction ofCaICS1andCaPALtranscripts and associated SA accumulation in resistant (WR315) chickpea after Foc1 inoculation [38]。By contrast,CaICS1,CaPALexpression and SA concentrations were significantly depleted at shoot tissues ofCaWRKY70overexpressing chickpea under Foc1 stress (Fig.4).EDS1 and PAD4 are two such important regulatory components of SA biosynthesis in plants upon pathogen stress [44,58]。In chickpea, we previously observed constant induction ofCaEDS1andCaPAD4transcripts at both shoot and root tissues of resistant genotypic plant under Foc1 infected condition [38]。However,CaEDS1andCaPAD4transcripts were downregulated atCaWRKY70overexpressing chickpea shoot tissues in response to Foc1 infection (Fig.5).This may strengthen negative regulatory role of CaWRKY70 in systemic defense reactions. SA signaling in plants depends on the activation of TGA transcription factors and NPR1. These two transcriptional modulators synergistically control expression of the two critical SA marker genes i.e.,PR1andPR5. The effective induction ofCaTGA1andCaTGA6mRNAs were observed at shoot tissues of Foc1 infected resistant chickpea, whereas susceptible plants were unable to stimulate such mRNA accumulation [38]。CaWRKY70 expresses at shoot tissues of susceptible chickpea after Foc1 inoculation and its overexpression in resistant chickpea plants markedly reducesCaTGA1andCaTGA6transcripts accumulation (Figs.1and5), which indicates to the impairment of conserved SA signaling in susceptible chickpea.CaNPR1,CaPR1andCaPR5transcripts follow the same pattern of Foc1 induced downregulation at shoot tissues ofCaWRKY70overexpressing chickpea than control transgenics (Fig.5).Importantly, CaWRKY70 represses the expression of SA and JA-marker genes i.e.,CaPR1,CaPR5andCaDefensinthat promotes susceptibility in transgenic chickpea (Fig.5).SA-mediated repression of JA-responsive gene expression is governed by cytosolic NPR1 [59]。WRKY70 controls JA-repressors based on cytosolic modification of NPR1 protein [59]。CaWRKY70-mediated suppression ofCaNPR1might play negative role in SA-responsiveCaPR1,CaPR5and JA-inducedCaDefensingene expression in chickpea (Fig.5).PDF1.2transcript inAtwrky70mutant was found to be low which enhanced uponB. cinereainfection. However, inCaWRKY70overexpressing chickpea,CaDefensinexpression has been downregulated under Foc1 infection [47]。Low levels of SA promotePRgenes expression in chickpea, whereas high concentrations inhibit both SA and JA signaling pathways. Thus, CaWRKY70 acts as an integrator of SA and JA responses in the regulation of chickpea defense responses to Foc1. Mutual antagonism and interaction between SA and JA pathways are common regulatory steps forCaWRKY70expression and its activation that governs wilt-disease resistance phenomenon in chickpea. Our previous study revealed that differential SAR induction in resistant and susceptible chickpea plants are analogous to the SA dependent gene expression and here, we convey mechanism of its attenuation. Hence, our present study explains the complexity of SA biosynthesis, signaling and its feed-back inhibition by CaWRKY70 that control Foc1 resistance/susceptibility in two contrasting chickpea accessions, respectively.
Regulatory sequences and DNA-binding activity of WRKY family members remarkably govern various cellular and stress responsive phenotypes in plants [60]。DNA binding role of CaWRKY70 is not an unusual phenomenon since, it is a nuclear localized protein (Additional file1: Figure S1). In Foc1 infected chickpea, CaWRKY70 inhibits the expression ofCaWRKY40signaling genes i.e.,CaWRKY33andCaMPK9(Figs.5and7).Recent finding suggests CaMPK9 interaction and phosphorylation provide stability to CaWRKY40 protein in chickpea upon Foc1 infection [43]。CaWRKY40 mediated upregulation ofCaMPK9expression was suppressed inCaWRKY70overexpressing chickpea. Shared transcriptional regulation ofAtWRKY18,AtWRKY40 andAtWRKY60 adjusts abscisic acid (ABA) signaling mediated abiotic stress responses in plants [61]。We found that CaWRKY70 binds atCaWRKY40cis-elements and represses its activity (Fig.6).有趣的是,CaWRKY40是ely regulates theCaMPK9promoter activation which has been established by both in vivo andin plantaexperiments (Fig.7).CaMPK9upstream elements deletion study also revealed its role in the modulation of promoter activity. CaWRKY70 activated transcription ofCaWRKY54gene in transgenic chickpea (Fig.5).These two transcription factors co-ordinately function as negative regulators of leaf senescence, stomatal closure, and osmotic stress tolerances inArabidopsis[41,62]。Although, our study demonstrated that CaWRKY70 and CaWRKY54 cooperatively contribute to Foc1 susceptibility in chickpea. CaWRKY70 mediated promoter modulation suggests bidirectional transcriptional regulation. Therefore, CaWRKY70 mediated inhibition of appropriate immune signaling in chickpea accomplishes through its direct and indirect negative regulatory influence on defense genes expression under Foc1 stress condition.
Transcriptional responses behind SAR activation by WRKY proteins were previously established inArabidopsis[63,64]。However, the mechanism of its repression is not known. Present study shows that deactivation of SAR in pathogen-free systemic tissues of chickpea is mediated by CaWRKY70 upon Foc1 infection. Repressor activity of AtWRKY70 onSARD1gene expression regulates the balance between growth and defense inArabidopsis[19,20]。Such regulatory functions are yet to be demonstrated in chickpea. CaWRKY70 is transcribed and translated at susceptible chickpea shoots upon Foc1 infection (Fig.1).Since, SAR activation was prominent at shoot tissues of resistant chickpea [38], we monitoredCaWRKY70over-expression effect in this background. The overall suppression of SA signal transduction network confers Foc1 susceptibility inCaWRKY70overexpressing chickpea (Figs.4and5).Although, it is tempting to monitorCaWRKY70knock-down effect in susceptible chickpea background as future attempt.
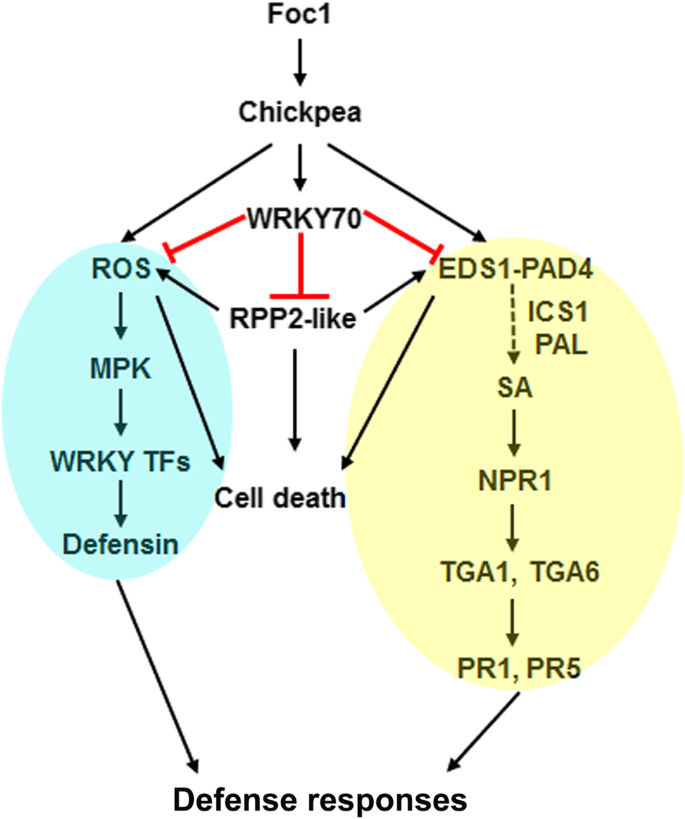
SA has immense roles in plant immunity, including resistance gene signaling. EDS1 is a well-known genetic regulator of SA production, resistance gene functioning and cell-death [65]。RPP2-like CC-NB-ARC-LRR protein and CaWRKY64 mount EDS1 dependent ectopic defense activation and cell-death in chickpea [30]。EDS1 mostly confers resistance as part of the TIR-NB-LRR signaling [66]。Based on our protein-protein interaction studies, we establish that physical interaction between CaWRKY70 and chickpea RPP2-like CC-NLR protein effectively suppresses ROS accumulation and cell-death inductionin planta(Fig.8).RPP4-mediated resistance response againstHyaloperonospora parasiticaEmoy2 was partially reduced inAtwrky70mutants [32]。On the other hand, RPP7-triggered defense reaction was not affected in this mutant background. Thus, R-protein mediated defense response pathway is positively correlated with AtWRKY70 functioning inArabidopsis, whereas it is inhibited by CaWRKY70 in chickpea might contradict WRKY70 dependent R-protein signaling in general. CaWRKY70 mediated inhibition of ROS accumulation in transgenic chickpea root, stem and leaves also support our interpretation (Fig.3).Although, reduction in oxidative bursts signaling and SA production does not fully correlate with the increased ion-leakage and fungal biomass accumulation inCaWRKY70overexpressing chickpea root than control transgenics (Figs.2and3).We reasoned that electrolyte leakage is associated with membrane damage due to higher colonization of Foc1 biomass, whereas ROS accumulation induced cell death is a defense phenomenon that inhibitsin plantaFoc1 growth. Importantly, less cell death promotes higher Foc1 colonization inCaWRKY70overexpressing chickpea roots. On the other hand, complete inhibition of EDS1 signaling not only affects SA induction, but also cell-death promotion (Figs.5and8).In summary, CaWRKY70 functions as nodal suppressor that includes fundamental defense regulators like, SA response and EDS1 into R-protein mediated signal transduction pathways highlighted in chickpea upon Foc1 stress (Fig.9).
Conclusions
Finally, this study provides information which may fill the gaps between already available knowledge about CaWRKY70 mediated transcriptional control of downstream defense signaling pathways. Promoter occupancy and protein-protein interaction play crucial roles for suppression of chickpea defense to Foc1. Interpretation of our findings may be translated and recapitulated for serial examination of multiple layers of defense signaling in chickpea. Repressor role of CaWRKY70 in modulating ROS homeostasis, SA biosynthesis and signaling is an interesting finding. Interconnection between several signaling cues in turn confer resistance against Foc1 in definite ways depending on time point of infection, duration, and severity. Notably, spatiotemporal expression patterns ofCaWRKY70mediated immune signaling elements renovates our apprehension. Thus, present study is useful to develop strategies for protecting chickpea fromFusariumwilt disease.
Methods
Plant materials and growth conditions
Experiments were performed using two different genotypes of chickpea (Cicer arietinumL.) i.e., JG62 (wilt susceptible) and WR315 (wilt resistant) obtained from Dr. Suresh C. Pande, ICRISAT (International Crops Research Institute for Semi-Arid Tropics), Patancheru, Andhra Pradesh, India. Surface sterilized seeds of both genotypes were sown in the pots containing autoclaved mixture of soil-rite and soil under natural greenhouse conditions at 22 to 25 °C temperature, 70% relative humidity, 100 μ mol m− 2 s− 1light intensity and 16 h photoperiod. Pots were watered from bottom at every 2 days and supplemented with half strength Hoagland’s medium (TS1094, Hi-media Laboratories, Mumbai, India).Nicotiana tabacumL. cv. Samsun NN andN. benthamianaseeds were gifted by Dr. Nrisingha Dey, Institute of Life science, Bhubaneshwar, India. Seeds were surface sterilized and grown aseptically on MS medium at 24 °C temperature and 60% relative humidity with light intensity of 100 μ mol m− 2 s− 1under 16 h photoperiod.
Fungal inoculation
F. oxysporumf. sp. ciceriRace1 (Foc1) fungal strain was obtained from Dr. Suresh C. Pande, International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, Andhra Pradesh, India. Sixteen-days-old chickpea plants were inoculated with Foc1 using sick-soil method according to the previously described method [34,43]。
Hormone treatment
For inducer treatments, 2 mM salicylic acid (SA, Hi-media), 100 μM ABA (Abscisic acid, Hi-media) and 50 μM JA (Jasmonic acid, Hi-media) was sprayed on greenhouse gown sixteen-days-old chickpea plants of both susceptible and resistant accessions. Leaf tissues were collected at 6 h of treatment for RNA isolation.
RNA isolation and quantitative real-time PCR (qRT-PCR) analyses
Shoot tissues of sixteen-days-old susceptible and resistant chickpea plants were collected and frozen in liquid nitrogen. Total cellular RNA was extracted from frozen sample using TRIZOL reagent (Himedia). First strand cDNA synthesis was carried out from 5 μg total RNA using First Strand cDNA synthesis Kit (Thermo Scientific, USA) following manufacturer’s guideline. qRT-PCR was performed using Bio-Rad iCycler (Bio Rad iQ5) with SyBr green (Bio Rad). The reaction mix containing SyBr green qPCR Super Mix (2×) (Bio Rad), 25 ng cDNA, and 0.3 μM of forward and reverse primers (Additional file3: Table S2). ChickpeaGlyceraldehyde-3-phosphate dehydrogenase(CaGAPDH) expression was used as internal control. Normalized fold change levels for all the genes were calculated using the 2−ΔΔ(Ct)method [67]。
Subcellular localization
For subcellular localization study, YFP gene was cloned inBamHI/SacI site of pBI121binary plant transformation vector. Full-lengthCaWRKY70gene was PCR amplified and fused in frame to N terminal part of the yellow fluorescent protein (YFP) gene in pBI121 vector for preparation of the35S:WRKY70-YFPconstruct. The vectors were mobilized into the competentAgrobacterium tumefaciensstrain GV3101.35S:WRKY70-YFPand control35S:YFPvectors were transiently transformed into onion epidermal cells byAgrobacterium. At 48 h post-agroinfiltration, epidermal cells were observed under a confocal microscope (Leica TCS SP2 AOBS system) to monitor localization patterns of fusion proteins under excitation and emission at 514 nm and 527 nm, respectively.
Molecular cloning
Full-length coding sequence ofCaWRKY70(GenBank Accession No. XM_004502763.3) was amplified from chickpea cDNA pool by reverse-transcriptase polymerase chain reaction (RT-PCR) using gene specific primers (Additional file3: Table S2). Purified PCR amplicons were restriction digested withBamHI/XhoI (Roche, Mannheim, Germany) and cloned in modified pBI221 vector containing short multi-cloning sites (MCS) region by replacingGUSgene. Cassette was gel excised after treatment withHindIII/EcoRI (Roche, Mannheim, Germany) and cloned in MCS of binary plant expression vector pCAMBIA2301. Cloning was checked by restriction digestion, which is followed by sequencing of the full-length gene. Binary plant transformation vector containingCaWRKY70gene was mobilized toAgrobacterium tumefaciensstrain AGL-1. Empty pCAMBIA2301 vector was also transformed intoAgrobacteriumstrain AGL-1 was used as vector control.
Chickpea transformation
Agrobacterium介导的鹰嘴豆转换as described by [68] with a modified rooting protocol [69]。Briefly, chickpea transformation was carried out with single cotyledon and half-embryo explant followed by infection withAgrobacteriumstrain AGL-1 harbouring empty pCAMBIA2301 vector and modified vector carryingCaWRKY70gene. Multiple shoots were regenerated from the explants and elongated with 0.25 mg/ l IAA (Indole-3-acetic acid) for 10 days. The elongated shoots were transferred to rooting medium (1/2 MS salts, B5 vitamins, 1 mg/ l IBA and 20 g/ l sucrose) [70]。Finally, rooted plantlets were properly hardened, transferred to glasshouse and established in the pots. Empty pCAMBIA2301 vector generated plants were used as control transgenics whereas,CaWRKY70gene carrying modified vector transformed plants were considered as overexpressing chickpea.
Disease intensity index
Disease intensity index was determined on control transgenic andCaWRKY70overexpressing chickpea based on the development of foliar symptoms at 0, 3, 7 and 12 dpi, respectively. Incidence of foliar symptoms (I) was set at 0 to 1 scale and disease severity (S) rated on a 0 to 4 scale (0 - no wilting; 1 - less wilting; 2 - partial wilting; 3 - wilting; 4 - severe wilting). Disease intensity index (DII) was calculated as DII = (I × S)/4 [71]。
Incidence of dead plants
Development of Foc1 infection on control transgenic andCaWRKY70overexpressing chickpea in the greenhouse experiment was recorded as incidence of dead plants at 0, 3, 7 and 12 dpi. The percentage of incidence of dead plants from transgenic chickpea was measured using the formula i.e., percentage of incidence of dead plants = (total number of infected plants/ total number of plants assessed) × 100 [72]。
Chlorophyll estimation
For chlorophyll extraction, one gram of control transgenic andCaWRKY70overexpressing chickpea leaf samples were crushed with 2 ml dimethyl sulfoxide (DMSO): acetone (1:1vol/ vol) mix. The samples were then kept in a refrigerator at 4 °C for 4 h. The samples were then centrifuged at 500 rpm for 5 min. Following this, supernatant was transferred to fresh 2 ml eppendorf tubes. The colour absorbance (A) of extracts was determined using Shimadzu UV 1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) at 645 and 663 nm wavelength against the blank solvent containing 80% acetone. Chlorophyll A and B content was estimated based on [73]。
DAB staining
H2O2accumulation in treatedCaWRKY70overexpressing chickpea root, stem and leaves were visualized by 3, 3′-diaminobenzidine (DAB) staining, according to the method of [74]。The plant tissues were immersed in 1 mg/ ml DAB (3, 3′-diaminobenzidine) solution (pH − 3.8) and vacuum infiltrated for 2 h followed by incubation of 8 h at room temperature. Chlorophyll was removed by incubating in 96% ethanol for overnight and photographed with a digital camera. DAB stained samples were oven dried for 24 h and crushed with sterile double distilled water to measure their intensities by spectrophotometer at 500 nm wavelength and water as blank.
Trypan blue staining
For trypan blue staining, lactophenol-trypan blue solution was prepared by mixing 10 ml lactic acid, 10 ml glycerol, 10 ml distilled water, 10 g of phenol and 10 mg of trypan blue. H2O2treated and Foc1 infected control and transgenic chickpea root, stem and leaves were subjected to trypan blue staining. Plant tissues soaked in trypan blue solution were warmed in a boiling water bath for 1 min and cleared with saturated chloral hydrate solution (2.5 g chloral hydrate dissolved in 1 ml distilled water) for 10 min. Decolorized plant samples were photographed. Trypan blue stained samples were dried and extracted with sterile double distilled water. The crude extracts were quantified using spectrophotometer at 500 nm wavelength against water as blank.
Bacterial expression and protein purification
PCR amplified full-lengthCaWRKY40andCaWRKY70genes were inserted intoEcoRI/XhoI site of pET28a(+)(Novagen, Germany) and transformed intoEscherichia coliBL21 (DE3) cells. Recombinant WRKY40 protein purification was carried out as previously described by [42]。Histidine tagged WRKY70 protein purification was performed according to previously described method by [49]。Protein induction was carried out with 1 mmisopropyl thio-β-D-galactoside (IPTG) at 37 °C for 1 h with vigorous shaking (160 rpm) and cells were harvested by centrifugation at 8000 rpm 4 °C for 5 min. Hexa-histidine tagged WRKY70 protein was purified from cell lysate by Ni-NTA affinity chromatography (Qiagen).
Antibody production
Anti-WRKY70 polyclonal antibodies were raised in rabbits. Two rabbits were immunized for antibody production. Rabbits were injected on 5 occasions with recombinant hexa-histidine tagged WRKY70 protein of 1.5 mg concentrations at every 3-week intervals. Serum obtained from each immunized rabbit was tested 2-weeks after each injection. Pre-immune serum was collected from each animal. Approximately, 20–30 ml serum/ rabbit was obtained. The serum was affinity purified. Antibodies were used at a final dilution of 1:10,000 for immunoblotting experiments.
Protein extraction and immunoblotting
Total soluble protein extraction from sixteen-days-old control and Foc1 inoculated susceptible and resistant chickpea shoots were performed using an ice-cold protein extraction buffer (50 mM Tris−HCl pH 7.5, 100 mM NaCl, 1 mM DTT, 0.5% Triton X-100, 0.1% SDS and 10% glycerol) followed by the addition of protease inhibitor cocktail (ETDA-free, Roche). Protein concentration was measured by Bradford assay [75]。Approximately, 20 μg of total soluble protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and detected by western blotting with anti-WRKY70 polyclonal primary antibody and an anti-rabbit IgG conjugated to horseradish peroxidase secondary antibody (Sigma, A-6667).
Conductivity measurement assay
For conductivity measurement experiment, control transgenic andCaWRKY70overexpressing chickpea roots were subjected to Foc1 infection at various times and washed thoroughly with water. 200 milligram control and Foc1 infected chickpea roots were incubated overnight in sterile tubes filled with 20.0 ml distilled water. Following this, electrical conductivity of water was measured using an electrolyte meter at indicated time points.
Estimation of Foc1 biomass
Amount of Foc1 biomass was measured according to the previously described method [76]。Genomic DNA isolated from Foc1 inoculated root tissues of control transgenic andCaWRKY70over-accumulating chickpea was used as template for real-time PCR with 5.8S rDNA primers listed in (Additional file3: Table S2).
Measurement of relative water content (RWC)
RWC of vehicle transgenic andCaWRKY70overexpressing chickpea plants were determined by weighing method upon control treatment and Foc1 infection [38]。
SA estimation by high performance liquid chromatography (HPLC)
SA concentrations were determined by HPLC (Shimadzu, Japan) provided with two LC-10 pumps and a UV detector system SPD-10A [77]。Total SA was extracted from 200 mg control and Foc1 treated shoot tissues of transgenic chickpea. The samples were dissolved separately in 200 μl of running buffer (0.2 M NaOAc, pH 5.2, and 10% methanol) and injected in a C-18 HPLC column (4 μm, 250 × 4.6 mm, Phenomenex, USA). A two-pump linear gradient system was used for separation of methanolic plant extracts i.e., pump A contains 1% acetic acid and pump B was filled with acetonitrile. SA detection was carried out at 254 nm wavelength, 30 °C temperature with a flow rate of 0.8 ml/min. The data obtained were combined using Shimadzu Class VP series software. Samples were identified according to their respective retention time (Rt) of peaks and quantity was calculated in mg g− 1FW based on area of the peak and the values obtained for standard used.
In silico DNA-protein interaction study
The sequence of putative CaWRKY70 protein was retrieved from NCBI (National Centre for Biotechnology Information). Template search and three-dimensional structure prediction of CaWRKY70 (PDB ID: c2aydA) was performed by homology modelling using Phyre2 tool (http://www.sbg.bio.ic.ac.uk/phyre2) [78]。Validation of the generated model was further carried out by Ramachandran plot analysis using RAMPAGE server (http://mordred.bioc.cam.ac.uk/~rapper/rampage.php) [79]。Molecular docking analysis of CaWRKY70 with W-Box DNA was done by HADDOCK (High Ambiguity Driven protein-protein Docking,www.haddocking.org) web server [80]。B DNA model with W-Box element (TGAC) was constructed using 3D-DART webserver, which corrects the nucleotide according HADDOCK specification [81]。Protein and DNA models were subjected to molecular docking by uploading their respective PDB files using easy interface at the HADDOCK server. The W-Box element of modelled DNA and the “WRKYGQK” amino acid sequences present in CaWRKY70 transcription factor were selected as the active residues for docking. Passive residues were automatically selected surrounding the active residues. Finally, docked structure was illustrated and visualized using UCSF Chimera (https://www.cgl.ucsf.edu/chimera/) [82]。
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed according to the method previously described by [83] showing CaWRKY70 and CaWRKY40 binding atCaWRKY40andCaMPK9promoters via W-boxes, respectively. ChIP primers are listed in the (Additional file3: Table S2).
Electrophoretic mobility shift assay (EMSA)
In vitro DNA binding activity of 6X histidine tagged WRKY70 protein was performed using EMSA experiments. To prepare the DNA probes for EMSA, equimolar concentration of each sense and antisense oligonucleotide of respective DNA duplexes were mixed in a reaction buffer containing 40 mM Tris−HCl; pH 7.5, 20 mM MgCl2, 50 mM NaCl. The reaction mix was heated at 95 °C for 10 min and slowly cooled down to room temperature for annealing. DNA duplexes were run on 7% PAGE (polyacrylamide gel electrophoresis) and gel-purified, followed by end labelling with γ-32P and T4-polynucleotide kinase (NEB). The labeled probes were then purified using QIAquick Nucleotide Removal Kit (Qiagen). The binding assay was performed using His-tagged WRKY70 protein. Typically, the binding reaction contained 100–200 ng of purified protein, 10 ng of double-stranded synthetic oligonucleotides end-labeled with γ-32P in a binding buffer containing 20 mM HEPES, pH 7.5, 100 mM KCl, 0.2 mM EDTA, 1 mM DTT, 2 mM MgCl2, and 1 μg of poly dI-dC. The protein was pre-incubated in binding buffer for 5 min prior to the addition of probe for eliminating the risk of non-specific binding and the reaction mix was incubated for another 30 min at room temperature. The complexes were resolved in 0.5X TBE-5% PAGE at (4 °C, 100 V) for about 2 h. After electrophoresis, gels were dried and exposed to phosphorscreen for imaging in a phosphorimager (Typhoon, GE Healthcare). For competition assays, unlabelled duplexes were added during the incubation stage.
Protoplast transfection and trans-inhibition assay
Protoplasts fromNicotiana tabacumcv. Xanthi (Brad) cell suspension culture was isolated and electroporated according to previously described method by [84]。For trans-inhibition assay,CaWRKY40promoter region was cloned betweenHindIII/BamHI site by replacing CaMV35S promoter in YFP containing pBI121 vector to obtainpWRKY40:YFP. Next,p35S:WRKY70andpWRKY40:YFPvectors were co-transfected into protoplasts and incubated in dark for 48 h. Confocal microscopy was performed to monitor the promoter activity.
GUS assay
Histochemical GUS staining of transgenic tobacco seedlings were performed according to the protocol [85]。Briefly, the tissues were incubated in GUS staining solution containing 1 mM X-Gluc (Duchefa Biochemie, Netherlands), 100 mM sodium phosphate (pH 7.0), 2 mM potassium ferricyanide, 2 mM potassium ferrocyanide, 10 mM EDTA, and 0.1% Triton X-100 under dark conditions at 37 °C for overnight (16 h). After staining, tissues were de-stained in 75% ethanol and photographed with a digital camera.CaWRKY40(LOC101512877) andCaMPK9(LOC101496681) promoter activity was monitored through the GUS expression analysis.CaWRKY40andCaMPK9promoter was inserted betweenHindIII/BamHI site of pBI121 vector by replacing CaMV35S promoter upstream to theGUSgene. Plasmids were transformed intoA. tumefaciensstrain LBA4404.p35S:WRKY70orp35S:WRKY40(effector constructs) andpWRKY40:GUSorpCaMPK9:GUS(reporter constructs) were co-infiltrated into the ventral surface ofN. tabacumleaves and subjected to GUS activity assay at 2 days post-infiltration. 100 milligram of agro-infiltrated tobacco leaf discs were collected in a 1.5 ml micro-centrifuge tube and crushed with liquid nitrogen into fine powder. Five hundred microliter of GUS extraction buffer containing 50 mM NaHPO4, pH- 7.0, 10 mM 2-mercapto ethanol, 10 mM Na2EDTA, 0.1% SDS and 0.1% triton X-100 was added to grinded sample. The mixture was then centrifuged at 13,000 rpm for 15 min at 4 °C and the cleared supernatant was collected. Data was normalized to protein concentration as measured by Bradford method [75]。Ten microgram crude protein extracts were mixed with 100 μl of GUS assay solution 2 mM 4-methyl umbelliferyl-d-glucuronide (4-MU) in extraction buffer. The reaction was carried out at 37 °C for 60 min and stopped by addition of 0.2 M Na2CO3. One hundred microliter reaction mixture was used to measure the fluorescence of GUS enzymatic activity using spectro-fluorometer (Hitachi, F-7000) under excitation and emission at 365 nm 455 nm.
Agrobacteriummediated transient infiltration
Agrobacterium tumefaciensGV3101 cells harbouring plasmids were grown overnight at 28 °C in 25 ml Luria-Bertani-broth with antibiotics. Next day, bacterial cells were pelleted by centrifugation at 8000 rpm and dissolved in 20 ml of induction medium (50 mM MES, pH 5.6, 0.5% (W/V) Glucose, 1.7 mM NaH2PO4, 20 mM NH4Cl, 1.2 mM MgSO4, 2 mM KCl, 17 μM FeSO4and 70 μM CaCl2) in presence of 200 μM acetosyringone. The solution was incubated at 28 °C for 4 h in shaker incubator with rotation speed 160 rpm. After incubation, bacterial cells were pelleted, resuspended in 20 ml of agroinfiltration medium (10 mM MES and 10 mM MgCl2, pH值5.6)3 h 100μM acetosyringone OD600(0.4) and then infiltrated into the ventral surface of fully expanded 6-weeks-oldN. benthamianaleaves. In case of chickpea leaves, transient infiltration was carried out using bath-sonication method as previously described by [30]。
BiFC assay
For BiFC studies, full length CDS of theCaWRKY70andCaRPP2-likeCC-NB-ARC-LRRgene (GenBank accession XM_012712097.1) was cloned betweenBamHI/SalI site of pSPYCE and pSPYNE vectors to generateCaWRKY70-YFPC-ter.and CC-NB-ARC-LRR-YFPN-ter.. Full-lengthCaWRKY64gene (GenBank accession XM_004489016.3) was cloned withinSpeI/XhoI site of pSPYCE vector. The control and fusion plasmids were transformed intoAgrobacteriumstrain GV3101.Agrobacteriumcells carrying control and fusion plasmids were co-infiltrated into the abaxial-side ofNicotiana benthamianaleaves byAgrobacteriummediated transient transformation according to the previously described method [30]。48 h后post-infiltration, epidermal cells were peeled off and subjected to confocal microscopy for reconstitution of the YFP signal.
Co-immunoprecipitation assay
For co-IP experiment, c-myc epitope tagged CC-NB-ARC-LRR protein and WRKY70 were transiently co-expressed inN. benthamianaleaves byAgrobacteriumstrain GV3101. At 2 dpi, 1 g of leaf samples were collected and quickly frozen in liquid nitrogen. Total protein was extracted from the infiltrated leaves using 2.5 mL ice cold protein extraction buffer containing 50 mM HEPES pH -7.5, 150 mM NaCl, 400 mM sucrose, 10% glycerol, 10 mM EDTA, 1% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate and protease inhibitor cocktail (Cat# 9599, Sigma-Aldrich, St Louis, Mo, USA). Crude protein extracts were centrifuged at 14,000 rpm for 20 min at 4 °C. The supernatants were pre-cleared using protein A-agarose beads and incubated with 10 μl of anti-Myc (ab39688) or anti-WRKY70 antibody for 3 h at 4 °C in a rotary shaker. One hundred microliter protein A-agarose beads (Bio-Bharati life science) were added to the samples and incubated for additional 3 h at 4 °C. The bound proteins were separated by centrifugation at 14,000 rpm for 20 min at 4 °C. Next, the beads were washed three times with ice cold wash buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 10 mM EDTA and 0.1% Triton X-100 and protease inhibitor cocktail). Bound proteins were eluted from the beads by 100 μl 1X laemmli sample buffer containing 250 mM Tris-HCl (pH 6.8), 10% SDS (W/V), 0.5% bromophenol blue (W/V), 50% glycerol and 50 mM DTT in a boiling water bath for 10 min. Western blotting was performed with anti-c-Myc and anti-WRKY70 antibodies, which is followed by addition of the horseradish peroxidase conjugated secondary antibody.
Statistical analyses
For statistical differences, Student’st-test was performed at a significance level ofp < 0.05 come next to multiple comparison of means by Tukey’s post-hoc test.
Availability of data and materials
No large-scale global data or any database was created for the study. All data generated during this study are included in this article and additional files.
Abbreviations
- ABA:
-
Abscisic acid
- ARC:
-
Apaf-1, Resistance and CED4 (Caenorhabditis eleganscell death 4 protein)
- BiFC:
-
Bimolecular Fluorescence Complementation
- Ca:
-
Cicer arietinumL.
- CBB:
-
Coomassie Brilliant Blue
- CC:
-
Coiled coil
- ChIP:
-
Chromatin Immunoprecipitation
- DNA:
-
Deoxyribonucleic acid
- EDS1:
-
Enhanced Disease Susceptibility 1
- EMSA:
-
Electrophoretic Mobility Shift Assay
- ET:
-
Ethylene
- HA:
-
Haemagglutinin
- HR:
-
Hypersensitive response
- ICS1:
-
Isochorismate Synthase 1
- JA:
-
Jasmonic acid
- LRR:
-
Leucine rich repeat
- MPK:
-
Mitogen Activated Protein Kinase
- mRNA:
-
Messenger RNA
- NB:
-
Nucleotide binding
- NLR:
-
Nucleotide binding oligomerization domain leucine rich repeat
- NPR1:
-
Non-expressor of PR1
- PAD4:
-
Ph值ytoalexin Deficient 4
- PAGE:
-
Polyacrylamide Gel Electrophoresis
- PAL:
-
Ph值enylalanine ammonia-lyase
- PCR:
-
Polymerase chain reaction
- PDB:
-
Protein Data Bank
- PR1:
-
Pathogenesis Related 1
- qRT-PCR:
-
Quantitative real-time PCR
- RNA:
-
Ribonucleic acid
- RPP2:
-
Recognition ofPeronospora Parasitica2
- SA:
-
Salicylic acid
- SDS:
-
Sodium Dodecyl Sulfate
- YFP:
-
Yellow Fluorescent Protein
References
- 1.
Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–9.https://doi.org/10.1038/nature05286.
- 2.
Jones JDG, Vance RE, Dangl JL. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354(6316).https://doi.org/10.1126/science.aaf6395.
- 3.
Ülker B, Somssich IE. WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol. 2004;7:491–8.https://doi.org/10.1016/j.pbi.2004.07.012.
- 4.
L胡Y,陈、王H,张L,王F,余D。Arabidopsistranscription factor WRKY8 functions antagonistically with its interacting partner VQ9 to modulate salinity stress tolerance. Plant J. 2013;74:730–45.https://doi.org/10.1111/tpj.12159.
- 5.
Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY super family of plant transcription factors. Trends Plant Sci. 2000;5:199–206 PMID: 10785665.
- 6.
Despres C, Subramaniam R, Matton DP, Brisson N. The activation of the potato PR-l0a gene requires the phosphorylation of the nuclear factor PBF-1. Plant Cell. 1995;7:589–98.https://doi.org/10.1105/tpc.7.5.589.
- 7.
Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 1996;15:5690–700 PMID: 8896462.
- 8.
Yu D, Chen C, Chen Z. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell. 2001;7:1527–40 PMID:11449049.
- 9.
Li J, Brader G, Palva ET. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate mediated signals in plant defense. Plant Cell. 2004;16:319–31.https://doi.org/10.1105/tpc.016980.
- 10.
Zheng Z, Qamar SA, Chen Z, Mengiste T.ArabidopsisWRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006;48:592–605.https://doi.org/10.1111/j.1365-313X.2006.02901.x.
- 11.
van Verk MC, Bol JF, Linthorst HJ. WRKY transcription factors involved in activation of SA biosynthesis genes. BMC Plant Biol. 2011;11:89.https://doi.org/10.1186/1471-2229-11-89.
- 12.
Tsuda K, Mine A, Bethke G, Igarashi D, Botanga CJ, Tsuda Y, et al. Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity inArabidopsis thaliana. PLoS Genet. 2013;9(12):e1004015.https://doi.org/10.1371/journal.pgen.1004015.
- 13.
Bigeard J, Colcombet J, Hirt H. Signaling mechanisms in pattern triggered immunity (PTI). Mol Plant. 2015;8:521–39.https://doi.org/10.1016/j.molp.2014.12.022.
- 14.
Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, et al. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–50.https://doi.org/10.1126/science.266.5188.1247.
- 15.
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–19.https://doi.org/10.1105/tpc.8.10.1809.
- 16.
Sticher L, Mauch-Mani B, Metraux JP. Systemic acquired resistance. Annu Rev Phytopathol. 1997;35:235–70.https://doi.org/10.1146/annurev.phyto.35.1.235.
- 17.
Vlot AC, Dempsey DMA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206.https://doi.org/10.1146/annurev.phyto.050908.135202.
- 18.
吴Wildermuth MC, Dewdney J, G, Ausubel调频。Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–5.https://doi.org/10.1038/35107108.
- 19.
Wang L, Tsuda K, Truman W, Sato M, Nguyen V, Katagiri F, et al. CBP60g and SARD1 play partially redundant critical roles in salicylic acid signaling. Plant J. 2011;67:1029–41.https://doi.org/10.1111/j.1365-313X.2011.04655.x.
- 20.
Zhou M, Lu Y, Bethke G, Harrison BT, Hatsugai N, Katagiri F, et al. WRKY70 prevents axenic activation of plant immunity by direct repression of SARD1. New Phytol. 2018;217(2):700–12.https://doi.org/10.1111/nph.14846.
- 21.
Jiang CH, Huang ZY, Xie P, Gu C, Li K, Wang DC, et al. Transcription factors WRKY70 and WRKY11 served as regulators in rhizobacteriumBacillus cereusAR156-induced systemic resistance toPseudomonas syringaepv. Tomato DC3000 inArabidopsis. J Exp Bot. 2016;67:157–74.https://doi.org/10.1093/jxb/erv445.
- 22.
Popescu SC, Popescu GV, Bachan S, Zhang Z, Gerstein M, Snyder M, et al. MAPK target networks inArabidopsis thalianarevealed using functional protein microarrays. Genes Dev. 2009;23:80–92.https://doi.org/10.1101/gad.1740009.
- 23.
Pitzschke A. Modes of MAPK substrate recognition and control. Trends Plant Sci. 2015;20:49–55.https://doi.org/10.1016/j.tplants.2014.09.006.
- 24.
Shetty NP, Jørgensen HJL, Jensen JD, Collinge DB, Shetty HS. Roles of reactive oxygen species in interactions between plants and pathogens. Eur J Plant Pathol. 2008;121:267–80.https://doi.org/10.1007/s10658-008-9302-5.
- 25.
博尔维尔医生、Daudi a . pl的活性氧ant pathogen interactions. In: del Rio LA, Puppo A, editors. Reactive oxygen species in plant signaling, signaling and communication in plants. Berlin: Springer-Verlag; 2009. p. 113–33.
- 26.
Torres MA, Dangl JL, Jones JDG.Arabidopsisgp91phoxhomologuesAtrbohDandAtrbohFare required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci. 2002;99:517–22.https://doi.org/10.1073/pnas.012452499.
- 27.
足立H, Nakano T, Miyagawa N, Ishihama N, Yoshioka M, Katou Y, et al. WRKY transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase inNicotiana benthamiana. Plant Cell. 2015;27:2645–63.https://doi.org/10.1105/tpc.15.00213.
- 28.
Chen L, Zhang L, Yu D. Wounding-induced WRKY8 is involved in basal defense inArabidopsis. Mol Plant Microbe Interact. 2010;23:558–65.https://doi.org/10.1094/MPMI-23-5-0558.
- 29.
Chakraborty J, Jain A, Mukherjee D, Ghosh S, Das S. Functional diversification of structurally alike NLR proteins in plants. Plant Sci. 2018a;269:85–93.https://doi.org/10.1016/j.plantsci.2018.01.008.
- 30.
Chakraborty J, Priya P, Dastidar SG, Das S. Physical interaction between nuclear accumulated CC-NB-ARC-LRR protein and WRKY64 promotes EDS1 dependentFusariumwilt resistance in chickpea. Plant Sci. 2018b;276:111–33.https://doi.org/10.1016/j.plantsci.2018.08.008.
- 31.
Sarris PF, Duxbury Z, Huh SU, Ma Y, Segonzac C, Sklenar J, et al. A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell. 2015;161(5):1089–100.https://doi.org/10.1016/j.cell.2015.04.024.
- 32.
Knoth C, Ringler J, Dangl JL, Eulgem T.ArabidopsisWRKY70 is required for full RPP4-mediated disease resistance and basal defense againstHyaloperonospora parasitica. Mol Plant Microbe Interact. 2007;20:120–8.https://doi.org/10.1094/MPMI-20-2-0120.
- 33.
Gurjar G, Barve M, Giri A, Gupta V. Identification of Indian pathogenic races ofFusarium oxysporumf. spciceriswith gene specific, ITS and random markers. Mycologia. 2009;101:484–95 PMID: 19623928.
- 34.
Gupta S, Chakraborti D, Rangi RK, Basu D, Das S. A molecular insight into the early events of chickpea (Cicer arietinum) andFusarium oxysporumf. sp.ciceri(该消灭)交互通过cDNA-AFLP分析。Ph值ytopathology. 2009;99:1245–57.https://doi.org/10.1094/PHYTO-99-11-1245.
- 35.
Gupta S, Bhar A, Chatterjee M, Das S.Fusarium oxysporumf. sp.cicerirace 1 induced redox state alterations are coupled to downstream defense signaling in root tissues of chickpea (Cicer arietinumL.). PLoS One. 2013;8:e73163.https://doi.org/10.1371/journal.pone.0073163.
- 36.
Sharma KD, Chen W, Muehlbauer FJ. Genetics of chickpea resistance to five races of fusarium wilt and a concise set of race differentials forFusarium oxysporumf. sp.ciceris. Plant Dis. 2005;89:385–90.https://doi.org/10.1094/PD-89-0385.
- 37.
Jimenez-Díaz RM, Castillo P, Jimenez-Gasco MM, Landa BB, Navas-Cortes JA. Fusarium wilt of chickpeas: biology, ecology and management. Crop Prot. 2015;73:16–27.https://doi.org/10.1016/j.cropro.2015.02.023.
- 38.
Bhar A, Chatterjee M, Gupta S, Das S. Salicylic acid regulates systemic defense signaling in chickpea duringFusarium oxysporumf. sp.cicerirace 1 infection. Plant Mol Biol Rep. 2018;36:162–75.https://doi.org/10.1007/s11105-018-1067-1.
- 39.
Li J, Brader G, Kariola T, Palva ET. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006;46:477–91.https://doi.org/10.1111/j.1365-313X.2006.02712.x.
- 40.
Mauch-Mani B, Slusarenko AJ. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance ofArabidopsistoPeronospora parasitica. Plant Cell. 1996;8:203–12.https://doi.org/10.1105/tpc.8.2.203.
- 41.
Li J, Besseau S, Toronen P, Sipari N, Kollist H, Holm L, et al. Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture inArabidopsis. New Phytol. 2013;200:457–72.https://doi.org/10.1111/nph.12378.
- 42.
Chakraborty J, Ghosh P, Sen S, Das S. Epigenetic and transcriptional control of chickpea WRKY40 promoter activity underFusariumstress and its heterologous expression inArabidopsisleads to enhanced resistance against bacterial pathogen. Plant Sci. 2018c;276:250–67.https://doi.org/10.1016/j.plantsci.2018.07.014.
- 43.
Chakraborty J, Ghosh P, Sen S, Nandi AK, Das S. CaMPK9 increases the stability of CaWRKY40 transcription factor which triggers defense response in chickpea uponFusarium oxysporumf. sp.ciceriRace1 infection. Plant Mol Biol. 2019;100:411–31.https://doi.org/10.1007/s11103-019-00868-0.
- 44.
Feys BJ, Moisan LJ, Newman MA, Parker JE. Direct interaction between theArabidopsis抗病信号蛋白,EDS1和PAD4. EMBO J. 2001;20:5400–11.https://doi.org/10.1093/emboj/20.19.5400.
- 45.
Saleh A, Withers J, Mohan R, Marqués J, Gu Y, Yan S, et al. Posttranslational modifications of the master transcriptional regulator NPR1 enable dynamic but tight control of plant immune responses. Cell Host Microbe. 2015;18(2):169–82.https://doi.org/10.1016/j.chom.2015.07.005.
- 46.
Wang J, Tao F, An F, Zou Y, Tian W, Chen X, et al. Wheat transcription factorTaWRKY70is positively involved in high-temperature seedling plant resistance toPuccinia striiformisf. sp.tritici. Mol Plant Pathol. 2017;18(5):649–61.https://doi.org/10.1111/mpp.12425.
- 47.
AbuQamar S, Chen X, Dhawan R, Bluhm B, Salmeron J, Lam S, et al. Expression profiling and mutant analysis reveals complex regulatory networks involved inArabidopsisresponse toBotrytisinfection. Plant J. 2006;48:28–44.https://doi.org/10.1111/j.1365-313X.2006.02849.x.
- 48.
Hu Y, Dong Q, Yu D. Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogenPseudomonas syringae. Plant Sci. 2012;185–186:288–97.https://doi.org/10.1016/j.plantsci.2011.12.003.
- 49.
Sen S, Chakraborty J, Ghosh P, Basu D, Das S. Chickpea WRKY70 regulates the expression of a homeodomain-leucine zipper (HD-zip) I transcription factorCaHDZ12, which confers abiotic stress tolerance in transgenic tobacco and chickpea. Plant Cell Physiol. 2017;58(11):1934–52.https://doi.org/10.1093/pcp/pcx126.
- 50.
Chen C, Chen Z. Isolation and characterization of two pathogen- and salicylic acid-induced genes encoding WRKY DNA binding proteins from tobacco. Plant Mol Biol. 2000;42:387–96 PMID: 10794538.
- 51.
Dong J, Chen C, Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 2003;51:21–37 PMID: 12602888.
- 52.
Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, et al. Salicylic acid-independent enhanced disease susceptibility1 signaling inArabidopsisimmunity and cell death is regulated by the monooxygenase FMO1 and the nudix hydrolase NUDT7. Plant Cell. 2006;18:1038–51.https://doi.org/10.1105/tpc.105.039982.
- 53.
Brodersen P, Petersen M, Pike HM, Olszak B, Skov S, Ødum N, et al. Knockout ofArabidopsisaccelerated-cell-death11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes Dev. 2002;16:490–502.https://doi.org/10.1101/gad.218202.
- 54.
Yoshida S, Ito M, Nishida I, Watanabe A. Identifcation of a novel geneHYS1/CPR5that has a repressive role in the induction of leaf senescence and pathogen-defense responses inArabidopsis thaliana. Plant J. 2002;29:427–37 PMID: 11846876.
- 55.
Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 2002;5:325–31 PMID: 12179966.
- 56.
Manikandan R, Raguchander T.Fusarium oxysporumf. sp.lycopersiciretardation through induction of defensive response in tomato plants using a liquid formulation ofPseudomonas fluorescens(Pf1). Eur J Plant Pathol. 2014;140(3):469–80.https://doi.org/10.1007/s10658-014-0481-y.
- 57.
默赫HC, Gajbhiye VT,辛格G, G·乔改变metabolomic profile of selected metabolites and improved resistance ofCicer arietinum(L.) againstMeloidogyne incognita(Kofoid &. White) Chitwood following seed soaking with salicylic acid, benzothiadiazole or nicotinic acid. Acta Physiol Plant. 2015;37(7):1–12.https://doi.org/10.1007/s11738-015-1888-6.
- 58.
Rustérucci C, Aviv DH, Holt BF, Dangl JL, Parker JE. The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell-death pathway controlled by LSD1 inArabidopsis. Plant Cell. 2001;13(10):2211–24 PMID: 11595797.
- 59.
Spoel SH, et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell. 2003;15:760–70.https://doi.org/10.1105/tpc.009159.
- 60.
Khuman A, Arora S, Makkar H, Patel A, Chaudhary B. Extensive intragenic divergences amongst ancient WRKY transcription factor gene family is largely associated with their functional diversity in plants. Plant Gene. 2019;22:100222.https://doi.org/10.1016/j.plgene.2020.100222.
- 61.
Chen H, Lai Z, Shi J, et al. Roles of arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol. 2010;10:281.https://doi.org/10.1186/1471-2229-10-281.
- 62.
Besseau S, Li J, Palva ET. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence inArabidopsis thaliana. J Exp Bot. 2012;63:2667–79.https://doi.org/10.1093/jxb/err450.
- 63.
Jaskiewicz M, Conrath U, Peterhansel C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011;12:50–5.https://doi.org/10.1038/embor.2010.186.
- 64.
Banday ZZ, Nandi AK.Arabidopsis thalianaGLUTATHIONE-S-TRANSFERASE THETA 2 interacts with RSI1/FLD to activate systemic acquired resistance. Mol Plant Pathol. 2018;19:464–75.https://doi.org/10.1111/mpp.12538.
- 65.
朱Venugopal SC、宋RD Mandal可,年代,钱德拉-Shekara AC, et al. Enhanced disease susceptibility 1 and salicylic acid act redundantly to regulate resistance gene-mediated signalling. PLoS Genet. 2009;5:e1000545.https://doi.org/10.1371/journal.pgen.1000545.
- 66.
Bhattacharjee S, Halane MK, Kim SH, Gassmann W. Pathogen effectors targetArabidopsisEDS1 and alter its interactions with immune regulators. Science. 2011;334(6061):1405–8.https://doi.org/10.1126/science.1211592.
- 67.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR 2−ΔΔ(Ct)method. Methods. 2001;25(4):401–8.https://doi.org/10.1006/meth.2001.1262.
- 68.
Chakraborti D, Sarkar A, Das S. Efficient and rapid in vitro plant regeneration system for Indian cultivars of chickpea (Cicer arietinumL.). Plant Cell Tiss Org Cult. 2006;86:117–23.https://doi.org/10.1007/s11240-005-9072-0.
- 69.
Chakraborty J, Sen S, Ghosh P, Sengupta A, Basu D, Das S. Homologous promoter derived constitutive and chloroplast targeted expression of syntheticcry1Acin transgenic chickpea confers resistance againstHelicoverpa armigera. Plant Cell Tiss Org Cult. 2016;125:521–35.https://doi.org/10.1007/s11240-016-0968-7.
- 70.
Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissues cultures. Physiol Plant. 1962;15:473–9.https://doi.org/10.1111/j.1399-3054.1962.tb08052.x.
- 71.
Landa BB, Navas-Cortés JA, Hervás A, Jiménez-Díaz RM. Influence of temperature and inoculum density ofFusarium oxysporumf. sp.cicerison suppression of fusarium wilt of chickpea by rhizosphere bacteria. Phytopathology. 2001;91:807–16.https://doi.org/10.1094/PHYTO.2001.91.8.807.
- 72.
Larkin RP, Fravel DR. Efficacy of various fungal and bacterial biocontrol organisms for control of fusarium wilt of tomato. Plant Dis. 1998;82(9):1022–8.https://doi.org/10.1094/PDIS.1998.82.9.1022.
- 73.
Arnon D. Copper enzymes in isolated chloroplasts, polyphenoxidase inBeta vulgaris. Plant Physiol. 1949;24:1–15.https://doi.org/10.1104/pp.24.1.1.
- 74.
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2in plants: H2O2accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–94.https://doi.org/10.1046/j.1365-313X.1997.11061187.x.
- 75.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.https://doi.org/10.1016/0003-2697(76)90527-3.
- 76.
Kariola T, Brader G, Li J, Palva ET. Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell. 2005;17:282e294.https://doi.org/10.1105/tpc.104.025817.
- 77.
Jain A, Singh S, Chaudhary A, Singh S, Singh HB. Modulation of nutritional and antioxidant potential of seeds and pericarp of pea pods treated with microbial consortium. Food Res Int. 2014;64:275–82.https://doi.org/10.1016/j.foodres.2014.06.033.
- 78.
Kelley LA, Sternberg MJE. Protein structure prediction on the web: a case study using the Phyre server. Nat Protoc. 2009;4:363–71.https://doi.org/10.1038/nprot.2009.2.
- 79.
Lovell SC, Davis IW, Arendall WB, de Bakker PI, Word JM, Prisant MG, et al. Structure validation by ca geometry: phi-psi and C-beta deviation. Proteins. 2003;50:437–50.https://doi.org/10.1002/prot.10286.
- 80.
de Vries SJ, van Dijk M, Bonvin AM. The HADDOCK web server for data-driven biomolecular docking. Nat Protoc. 2010;5:883–97.https://doi.org/10.1038/nprot.2010.32.
- 81.
Geer LY, Domrachev M, Lipman DJ, Bryant SH. CDART: protein homology by domain architecture. Genome Res. 2002;12:1619–23.https://doi.org/10.1101/gr.278202.
- 82.
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF chimera- a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–12.https://doi.org/10.1002/jcc.20084.
- 83.
Saleh A, Alvarez-Venegas R, Avramova Z. An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications inArabidopsisplants. Nat Protoc. 2008;3:1018–25.https://doi.org/10.1038/nprot.2008.66.
- 84.
Kumar D, Patro S, Ranjan R, Sahoo DK, Maiti IB, Dey N. Development of useful recombinant promoter and its expression analysis in different plant cells using confocal laser scanning microscopy. PLoS One. 2011;6(9):e24627.https://doi.org/10.1371/journal.pone.0024627.
- 85.
Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–7.3327686.
Acknowledgements
We are indebted to the Director, Bose Institute for providing infrastructural facilities. SD acknowledges Indian National Science Academy for providing Senior Scientist Fellowship. JC acknowledges ICAR, Govt. of India for providing fellowship. Authors sincerely thank Dr. Nrisingha Dey (ILS Bhubaneswar, India) for protoplast transformation experiment. Authors are thankful to Prof. Sudip Chattopadhyay (NIT Durgapur, India) and Dr. Anupama Ghosh (Bose Institute Kolkata, India) for kind gift of BiFC and mCherry vectors, respectively. Authors extended sincere thanks to Mr. Swarnava Das for his technical assistance. Mr. Sudipta Basu and Mr. Surajit Maity are duly acknowledged for their help in maintenance of the plants in glasshouses and other technical supports.
Funding
This project was partially supported by Indian Council of Agricultural Research (Grant No. NFBSFARA/AB-2010 (2010–11 dt. 24.01.2011). The funders were not involved in designing of the research, data collection, analysis and manuscript writing.
Author information
Affiliations
Contributions
JC and SD conceived and designed research. JC, SS, PG and AJ performed the experiments. JC, SS and SD analyzed the data and JC, PG, AJ and SD wrote the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1 Figure S1.
Subcellular localization of control YFP and CaWRKY70-YFP after transient expression in onion epidermal cells byAgrobacterium. Bars represent 250 μm. Red and white arrows indicate nucleus and cytoplasm, respectively.Figure S2.PCR amplification ofCaWRKY70gene cloned in pCAMBIA2301 vector after resolved on 1.2% agarose gel. Lane-M denotes DNA molecular weight marker in kilobases (kb). Lane-1, 2, and 3 show positive clones. Red arrows indicateCaWRKY70PCR amplicons.Figure S3.Homology modelling and Ramachandran plot calculation of CaWRKY70 protein.a,bandcPredicted structure of CaWRKY70 showing five anti-parallel β-strands.d,eQualitative assessment of stereo chemical and spatial arrangement of amino acids present on CaWRKY70 protein using RAMPAGE server.Figure S4.MUG assay quantitation of CaWRKY70 mediated reduction inpWRKY40-GUS activity. Plus (+) and minus (−) signs show presence or absence of the specific components. Error bars represent ±SD (n = 5). Asterisks (*) indicate values are different from one another in statistically significant manner as determined by Student’sttest (***P < 0.001).Figure S5.CaMPK9transcript accumulation in susceptible JG62 and resistant WR315 chickpea shoots under control treatment (0 dpi) and Foc1 infection (7 dpi) by real-time PCR.CaGAPDHmRNA level was used as internal control. Fold change was calculated relative to the control treatment. Error bars indicate ±SD of three biological replicates. Student’sttest was performed to determine its significance level as compared to the control treatment, **P ≤ 0.01 and ***P ≤ 0.001.
Additional file 2 Table S1.
Statistics of the top five clusters of CaWRKY70-DNA complex generated by HADDOCK server.
Additional file 3 Table S2.
List of primers used in this study.
Rights and permissions
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chakraborty, J., Sen, S., Ghosh, P.et al.Inhibition of multiple defense responsive pathways by CaWRKY70 transcription factor promotes susceptibility in chickpea underFusarium oxysporumstress condition.BMC Plant Biol20,319 (2020). https://doi.org/10.1186/s12870-020-02527-9
Received:
Accepted:
Published:
Keywords
- Immune response
- Protein-protein interaction
- Reactive oxygen species (ROS)
- R-protein signaling
- Systemic acquired resistance (SAR)
- Transcriptional regulation