- Research article
- Open Access
- Published:
Simultaneous induction of mutant alleles of two allergenic genes in soybean by using site-directed mutagenesis
BMC Plant Biologyvolume20, Article number:513(2020)
Abstract
Background
Soybean (Glycine max)是一个major protein crop, because soybean protein has an amino acid score comparable to that of beef and egg white. However, many allergens have been identified among soybean proteins. A decrease in allergenic protein levels would be useful for expanding the market for soybean proteins and processed foods. Recently, the CRISPR/Cas9 system has been adopted as a powerful tool for the site-directed mutagenesis in higher plants. This system is expected to generate hypoallergenic soybean varieties.
Results
We used two guide RNAs (gRNAs) andAgrobacterium-mediated transformation for simultaneous site-directed mutagenesis of two genes encoding the major allergens Gly m Bd 28 K and Gly m Bd 30 K in two Japanese soybean varieties, Enrei and Kariyutaka. We obtained two independent T0Enrei plants and nine T0Kariyutaka plants. Cleaved amplified polymorphic sequence (CAPS) analysis revealed that mutations were induced in both targeted loci of both soybean varieties. Sequencing analysis showed that deletions were the predominant mutation type in the targeted loci. TheCas9-free plants carrying the mutant alleles of the targeted loci with the transgenes excluded by genetic segregation were obtained in the T2and T3generations. Variable mutational spectra were observed in the targeted loci even in T2and T3progenies of the same T0plant. Induction of multiple mutant alleles resulted in six haplotypes in theCas9-free mutants derived from one T0plant. Immunoblot analysis revealed that no Gly m Bd 28 K or Gly m Bd 30 K protein accumulated in the seeds of theCas9-free plants. Whole-genome sequencing confirmed that aCas9-free mutant had also no the other foreign DNA from the binary vector. Our results demonstrate the applicability of the CRISPR/Cas9 system for the production of hypoallergenic soybean plants.
Conclusions
Simultaneous site-directed mutagenesis by the CRISPR/Cas9 system removed two major allergenic proteins from mature soybean seeds. This system enables rapid and efficient modification of seed components in soybean varieties.
Background
Soybean (Glycine max, 2n = 2x = 40) is one of the most important protein crops used for food and forage worldwide, because its seeds contain high-quality proteins with an amino acid score comparable to that of beef and egg white [1].Diverse soybean proteins are responsible for the physical properties of foods and other products made from soybean seeds [2,3].In the USA and Europe, 5 to 8% of babies and 2% of adults are allergic to soybean [4].Several subunits of major storage proteins such as 7S and 11S globulins and 2S albumin are representative soybean allergens [5].The vicilin-like glycoprotein Gly m Bd 28 K and the oil-body-associated protein Gly m Bd 30 K are also reported as major soybean allergens [6,7].Hydrophobic proteins Gly m 1A and Gly m 1B and the hull protein Gly m 2 are related to asthma outbreaks in Spain [8,9].Profilin Gly m 3 and the pathogenesis-related protein Gly m 4 are cross-reactive with antigens from other sources involved in sensitization and symptom induction [10,11].Positive response to soybean protein in allergic reaction has been reported in 14% of patients diagnosed with food allergies with atopic dermatitis [12].Therefore, development of hypoallergenic soybean varieties or establishment of a procedure to remove allergens would be useful for expanding the market of soybean proteins and processed foods.
Protein fractionation on the basis of the differences in protein solubility at different salt concentrations and pH can be used to characterize the biochemical and physical properties of proteins [13,14,15].This technique is also used for the removal of specific allergens from soy foods. Gly m Bd 30 K was efficiently removed from soy milk by acidifying it to pH 4.5 with 1 M Na2SO4[16].
大豆遗传改良是通过横sing plants carrying allergen-deficient alleles from soybean genetic resources or by mutagenesis to generate allergen-deficient mutant alleles. A number of spontaneous or induced mutants deficient in subunits of 7S or 11S globulins have been reported [17,18,19,20].Among the germplasm of wild soybean (G. soja), Hajika et al. [20] found one accession lacking the α-, α’-, and β-subunits of 7S globulin. The deficiency of these subunits is controlled by a single dominant gene (Scg-1),这是与post-transcrip密切相关tional gene silencing [21].To develop hypoallergenic soybean through crossing and subsequent back-crossing, this dominant gene has been introduced into an elite variety, Fukuyutaka [22].The soybean variety Yumeminori lacks α- and α’-subunits of 7S globulin, and Gly m Bd 28 K, and has a decreased level of the β-subunit of 7S globulin; this variety has been developed through mutagenesis by gamma-ray irradiation [23].Mutagenesis of the soybean variety VLSoy-2 by gamma-ray irradiation generated mutant lines lacking the A3-subunit of 11S globulin [24].This mutagenesis also produced plants lacking α- and α’-subunits of 7S globulin [24].Stacking of recessive mutant alleles of the genes for Kunitz trypsin inhibitor, agglutinin, and Gly m Bd 30 K was performed in the genetic background of the soybean variety Williams 82 [25].Proteome analysis revealed that the stacking of these mutant alleles markedly decreased the accumulation of these allergens [25].
The biotechnological approach can also help to decrease the accumulation of allergens in soybean seeds. Down-regulation of the gene encoding Gly m Bd 30 K greatly suppresses the accumulation of the targeted protein in seeds of transgenic soybean [26].The accumulation of α-, α’-, and β-subunits of 7S globulin in soybean seeds can be greatly decreased through RNA interference or artificial microRNA systems [27,28].Recently, the transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated endonuclease 9 (Cas9) systems have become the main platforms for site-directed mutagenesis in higher plants [29,30,31,32].They enable the fine tuning of traits in soybean breeding when applied to various soybean varieties. The CRISPR/Cas9 system can be used to develop hypoallergenic soybean directly from elite varieties, because it has been optimized for various soybean varieties [33,34,35,36,37].
The subunits of 7S and 11S globulins are closely associated with seed characteristics important for food processing such as gel-forming and emulsifying properties [38,39,40].To produce hypoallergenic soybeans without impairing the processing properties, we focused on two allergenic proteins, Gly m Bd 28 K and Gly m Bd 30 K, because no pyramiding of mutant alleles of these allergens in soybean has been reported. Here, we constructed a plasmid for simultaneous site-directed mutagenesis of these genes with the CRISPR/Cas9 system and used it forAgrobacterium-mediated transformation of two soybean varieties.Cas9-free plants carrying mutant alleles of the targeted loci, with the transgenes excluded by genetic segregation, were obtained in the T2or T3generations. Immunoblot analysis revealed that Gly m Bd 28 K and Gly m Bd 30 K proteins did not accumulate in seeds of theCas9-free plants. Our results demonstrate the applicability of the CRISPR/Cas9 system for the production of hypoallergenic soybean plants.
Results
Generation of transgenic soybean plants harboring the CRISPR/Cas9 expression module.
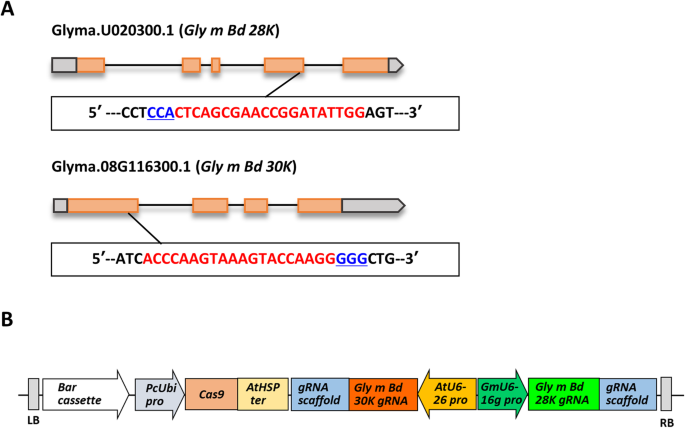
To conduct the site-directed mutagenesis of soybean with the CRISPR/Cas9 system, we designed two guide RNAs (gRNAs) to mutagenize theGly m Bd 28 KandGly m Bd 30 Kloci (Fig.1a). Explants of Enrei and Kariyutaka were inoculated withAgrobacteriumharboring the pMR284_28K_30K plasmid (Fig.1b). Two Enrei T0plants (E1 and E2) and nine Kariyutaka T0plants (K1 to K10) were obtained. All the T0plants set T1seeds. In our previous study, many T0plants produced by our soybean transformation system failed to transmit the transgenes into the T1progeny [41].This fact indicates that T0plants originated from chimeric tissues which contained transformed and non-transformed cells [41].Therefore, we did not examine transgene integration or the induction of mutagenesis in the T0plants, and grew representative T1plants for further analyses. The plant numbers of the T1and T2generations were indicated by giving the T0individual number followed by the branch number for each generation.
Targeted regions (a) and expression vector (b) of simultaneous site-directed mutagenesis of soybean genesGly m Bd 28 KandGly m Bd 30 Kby using the CRISPR/Cas9 system.aLocations of the targeted sites ofGly m Bd 28 KandGly m Bd 30 Kgenes. Boxes and pentagons, exons of the genes; bold lines, introns of the genes. Red and blue nucleotide sequences denote the gRNAs-targeted regions and the proto-spacer adjacent motif (PAM) regions, respectively.bVector structure of the CRISPR/Cas9 expression module for soybean transformation.Barcassette,Bar-marker gene unit;PcUbi pro, parsley (Petroselinum crispum) ubiquitin promoter;AtHSP ter, terminator of an Arabidopsis heat shock protein gene;AtU6 pro, Arabidopsis U6 promoter;GmU6–16 g pro, soybean U6–16 promoter
Mutations in T1plants detected by cleaved amplified polymorphic sequence (CAPS) analysis
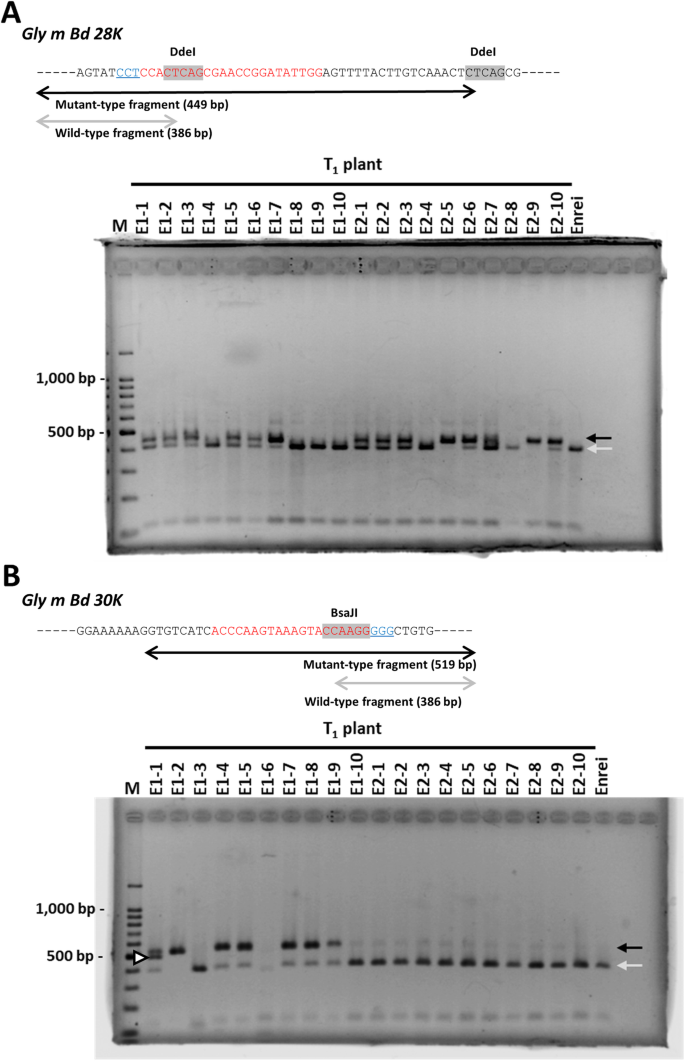
Representative 20 T1Enrei plants and 25 T1Kariyutaka plants derived from 12 T0plants were grown, and the induction of mutagenesis in the targeted loci was evaluated by CAPS analysis of the genomic DNA. The DNA fragments were classed into wild-type and mutant-type based on the expected size; a fragment of unexpected size was also detected and considered as mutant-type (Fig.2). Mutations were detected in both targeted loci in plants of both varieties (Fig.2, Additional file2: Figure S1, Additional file1: Tables S1, S2). Integration of the transgene was also examined by PCR analysis withCas9-specific primers. PCR analysis revealed that 11 T1plants (24.4% of all T1植物检验)回答eCas9-免费的;其中,E1-4、E1-8 E1-9突变alleles in theGly m Bd 30 Klocus, whereas the others had the wild-type alleles of both targeted loci (Additional file1: Tables S1, S2).
Confirmation of mutagenesis of targeted loci in representative Enrei-T1plants by CAPS analysis. The schematic diagrams above panels show DdeI or BsaJI restriction sites (shaded in gray) in fragments amplified with specific primers. Red and blue nucleotide sequences have the same meaning as those in Fig.1. Gray arrows, the sizes of expected wild-type fragments; black arrows, the sizes of expected mutant-type fragments; open triangle, a fragment of unexpected size considered as mutant type. M, molecular weight marker (100-bp ladder)
Transmission of mutations and transgenes to the T2generation
Because none of theCas9-free T1plants had mutations in both targeted loci, 13 representative T1plants were advanced to the next generation. A total of 348 T2seeds collected from the 13 T1plants were evaluated for the mutations in the targeted loci (Table1). In CAPS analysis, 227 (65%) T2seeds showed mutant-type fragments of both targeted loci (Table1, Additional file2: Figs. S2, S3). No mutations were detected in 20 T2seeds (Table1). Thus, frequency of simultaneous site-directed mutagenesis in both targeted loci was much higher in the T2generation than in the T1generation (Table1, Additional file1: Tables S1, S2).
Although theCas9gene was detected in all 13 T1plants (Additional file1: Tables S1, S2), it was removed by genetic segregation in 52 T2seeds (Table2, Additional file2: Fig. S4). Among them, 38 T2seeds had mutant alleles in one or both targeted loci (Table2). Of these 38 seeds, 14 seeds belonging to both varieties had mutant alleles in both targeted loci (double-mutants; Table2). Representative mutational spectra of the T2seeds are shown in Fig.3. The genotypes were divided into homozygous (theGly m Bd 28 Klocus in K4–1-37 andGly m Bd 30 Klocus in E1–5-17, K1–3-11, and K4–1-37 T2seeds), heterozygous (theGly m Bd 28 Klocus in E1–2-6 and E1–5-17, and K1–3-11 T2seeds), and biallelic mutant types (theGly m Bd 30 Klocus in E1–2-6) (Fig.3). Deletions were predominant in the mutational spectra of these T2seeds (Fig.3).
Mutational spectra of the targeted loci in double-mutant T2seeds. Red and blue nucleotide sequences have the same meaning as those in Fig.1. Green nucleotide denotes an insertion. Letters and numbers in parentheses indicate the type of mutation in the targeted locus: e.g.,d1, a single-nucleotide deletion;i1, a single-nucleotide insertion;wt, no mutation. Control, reference sequence (Enrei or Kariyutaka)
Development of double-mutant T3seeds
We used the heterozygous and biallelic mutants to develop more homozygous mutant alleles. We collected T3seeds and sequenced both targeted loci. In total, 4 haplotypes in Enrei and 21 haplotypes in Kariyutaka were found in the double-mutants (Table3). Deletions (1 to 43 nucleotides) were the most common mutations (Table3, Additional file2: Figure S5). Predicted amino acid sequences of Gly m Bd 28 K (Figure S6) and Gly m Bd 30 K (Figure S7) are shown in Additional file2. Three mutant alleles (d3andd6for theGly m Bd 28 Klocus, andd6for theGly m Bd 30 Klocus) had in-frame mutations (Additional file2: Figures S6, S7). In the Gly m Bd 30 K locus, the 3-nucleotide deletion generated a stop codon at the mutation site, and the 33-nucleotide deletion was not predicted as an in-frame mutation, because the deleted region contained the splicing site (Additional file2: Figure S7).
Analysis of Gly m Bd 28 K and Gly m Bd 30 K proteins in mature double-mutant seeds
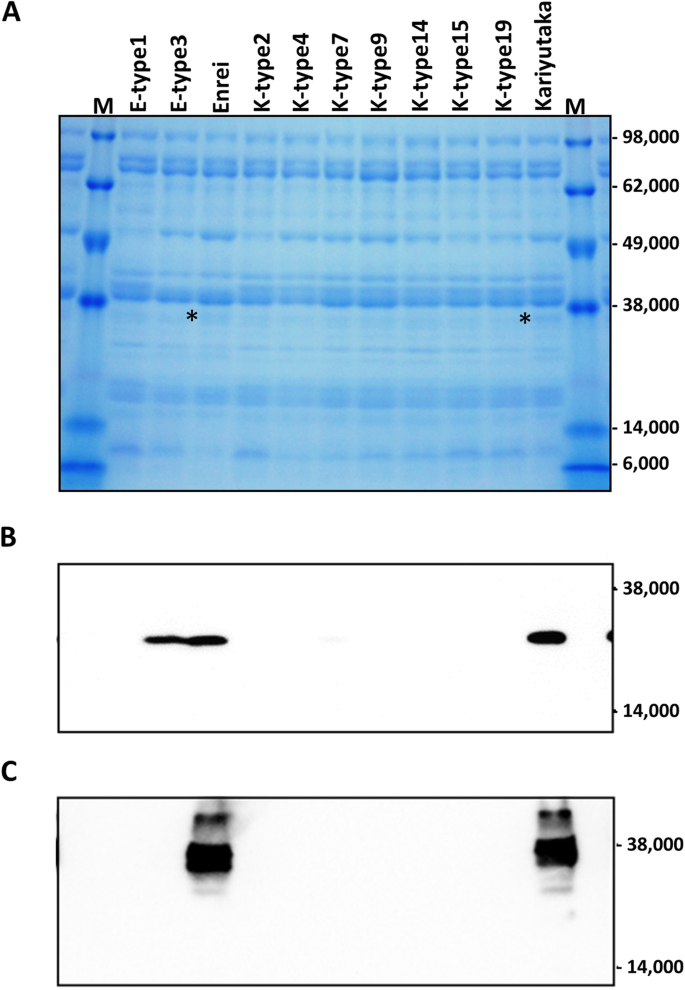
We selected two Enrei haplotypes (E-type1 and E-type3) and seven Kariyutaka haplotypes (K-type2, K-type4, K-type7, K-type9, K-type14, K-type15, and K-type19) from the double-mutants (Table3), and examined the composition of crude protein fractions prepared from mature seeds. The Gly m Bd 30 K protein was visually detectable in Enrei and Kariyutaka but not mutant seeds, whereas Gly m Bd 28 K was not detectable in any seeds in the SDS-PAGE analysis (Fig.4a). The double-mutant seeds had no signal bands that were not detected in wild-type seeds (Fig.4a). To detect the Gly m Bd 28 K and Gly m Bd 30 K proteins specifically, immunoblot analysis was conducted in double-mutant and wild-type seeds. In the immunoblot analysis, the Gly m Bd 28 K and Gly m Bd 30 K proteins were detected only in seeds of wild-type Enrei or Kariyutaka, except that Gly m Bd 28 K was also detected in the E-type3 haplotype (Fig.4b, c). No immunoreactive band of unexpected size was detected (Additional file2: Figure S8).
SDS-PAGE and immunoblot analyses of the crude proteins of representative double-mutant T3and wild-type mature seeds.aProteins separated by SDS-PAGE and stained with Coomasie Brilliant Blue. Designations of mutations are as in Table3. Asterisks denote signal of putative Gly m Bd 30 K protein. M, molecular weight marker.bImmunoblot analysis using polyclonal antibody against Gly m Bd 28 K protein.cImmunoblot analysis using polyclonal antibody against Gly m Bd 30 K protein. Gly m Bd 30 K protein. Images of full-length gel and blots are provided in additional file2(Figure S8)
Expression levels of theGly m Bd 28 Kand theGly m Bd 30 Kgenes
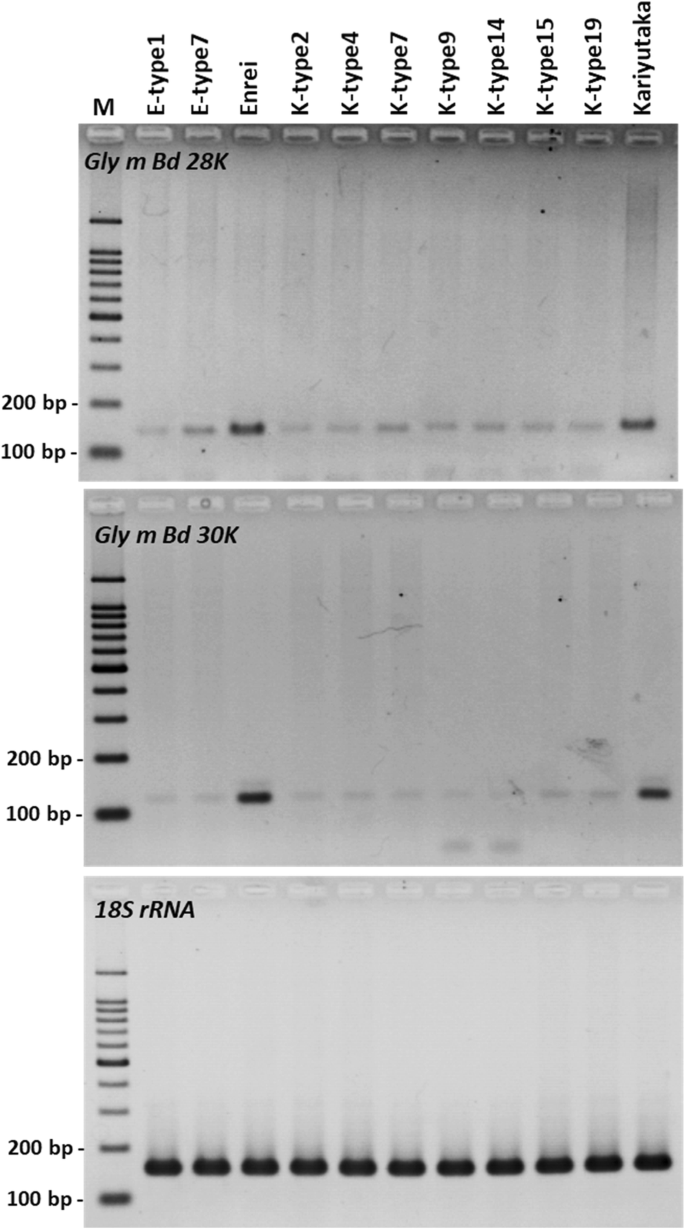
To evaluate the expression levels of theGly m Bd 28 Kand theGly m Bd 30 Kgenes, we extracted total RNA from the mature T3seeds of two Enrei mutants (E-type1 and E-type3), seven Kariyutaka mutants (K-type2, K-type4, K-type7, K-type9, K-type14, K-type15, and K-type19), Enrei and Kariyutaka, and conducted semi-quantitative RT-PCR analysis of the region up-stream of the mutation site (Additional file2: Figure S9). Although amplified products of the 18S ribosomal RNA (18S rRNA) were detected at similar levels in all mature seeds of the mutants, Enrei, and Kariyutaka (Fig.5), all mutants showed lower expression levels of theGly m Bd 28 KandGly m Bd 30 Kgenes than those of wild-type (Enrei and Kariyutaka) seeds (Fig.5).
Semi-quantitative RT-PCR of theGly m Bd 28 Kand theGly m Bd 30 Kgenes mature seeds of representative double-mutant T3and wild-type mature seeds. The expression level of targeted loci was evaluated based on the quantity amplified for18S rRNAas a control endogenous gene. PCR was performed at 38 cycles for theGly m Bd 28 Kand at 30 cycles for theGly m Bd 30 Kand18S rRNA
Whole-genome sequencing in T2plants to validate the absence of foreign DNA
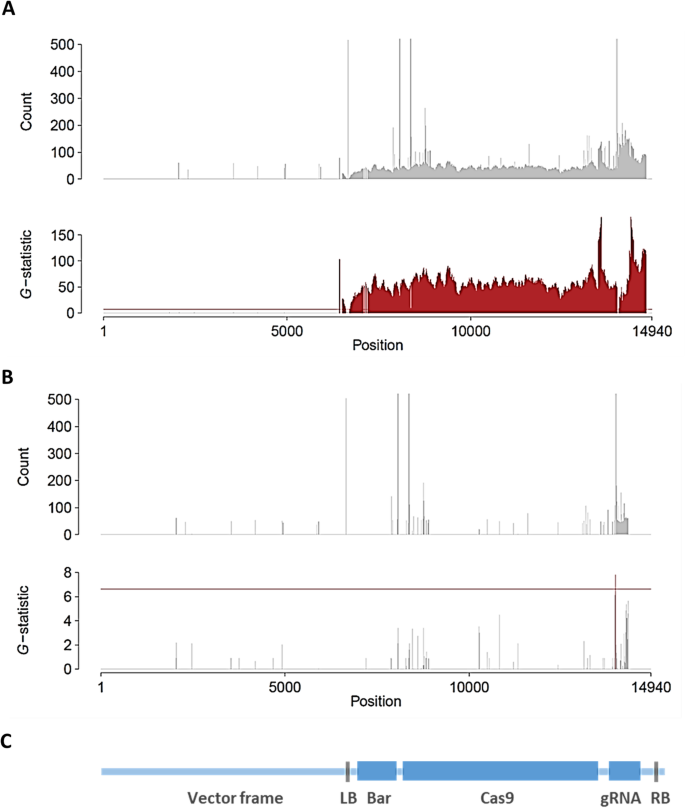
T2mutant plants K2–1-16 and K4–1-37 were selected for whole-genome sequencing analysis. These plants had homozygous mutant alleles in both loci. The K2–1-16 plant had mutant alleles with a single-nucleotide insertion in theGly m Bd 28 Kand a 2-nucleotide deletion in theGly m Bd 30 Kloci. The genome of K4–1-37 contained mutant alleles with a 6-nucleotide deletion in theGly m Bd 28 Klocus and a 2-nucleotide deletion in theGly m Bd 30 Klocus. PCR analysis detected the presence of theCas9gene in the genome of K2–1-16, whereas K4–1-37 wasCas9free. The whole genomes of the two T2plants were sequenced, and the presence of foreign DNA was examined by thek-mer detection method [42].Each 20-mer identical between the plant genome and the vector was detected (Fig.6). The genome of K2–1-16 clearly showed significant signals in a vector-wide manner (Fig.6a, c), whereas that of K4–1-37 had no signal of foreign DNA from the vector (Fig.6b). A significant signal found in theG统计的K4-1-37被认为是一个错误的订单sitive, because it had a much lower value than that of K2–1-16.
Detection of unintended remaining foreign DNA in T2plants. The counts ofk-mer andG-statistic are shown for (a) a transgenic sample K2–1-16 and (b) aCas9-free sample K4–1-37.cStructure of the binary expression vector pMR284_28K_30K. The horizontal axis indicates the nucleotide positions in the vector; horizontal red line is the 1% significance level byG-test.G-statistic values exceeding the 1% level of significance are in red. Thek-mer counts over 500 are omitted
Morphological characteristics of double mutants
To assess the consequences of the site-directed mutagenesis in the targeted loci, we examined the morphology of the mutant plants. Because many mutant alleles in the targeted loci were detected in the Kariyutaka T2generation (Fig.3), we examined the morphological characteristics of T2plant body and T3seed size and shape. No difference was detected between the double-mutants and wild-type in the plant and seed morphological characteristics (Additional file2: Figures S10, S11).
Discussion
Gly m Bd 28 K and Gly m Bd 30 K are the major allergenic proteins in soybean seeds [6,43].The mutant alleles of these loci have been identified by surveying the soybean germplasm or generated by gamma-ray irradiation mutagenesis [19,44,45], and stacking of these mutant alleles will enable development of hypoallergenic soybean lines. In contrast, site-directed mutagenesis mediated by the CRISPR/Cas9 system enables the induction of mutations directly in the targeted loci of the desirable donor plants such as varieties and elite breeding lines. This approach dramatically shortens breeding period and saves labor. In this study, we performed simultaneous site-directed mutagenesis of bothGly m Bd 28 KandGly m Bd 30 Kloci in two Japanese soybean varieties. A total of 14 T2-generation seeds possessed mutant alleles of both loci and had theCas9gene removed through genetic segregation (Table3). Among all mutations, deletions were predominant and caused frame-shifts (Additional file2: Figures S4–S6). The frame-shift mutations resulted in the deficiency in proteins recognized by the polyclonal antibodies against Gly m Bd 28 K and Gly m Bd 30 K proteins (Fig.4). No bands of unexpected size were detected with either of these antibodies (Fig.4). Frame-shift mutations in the targeted loci decreased the expression levels of theGly m Bd 28 Kand theGly m Bd 30 Kgenes (Fig.5). These findings suggest that the frame-shift mutations produce aberrant mRNAs from the targeted locus, which induced nonsense mRNA decay (NMD), like in a site-directed mutagenesis study conducted inBrassica carinatausing the hairy root transformation system [46].The lower expression level than wild-type might result in the deficiency in proteins recognized by the polyclonal antibodies against Gly m Bd 28 K and Gly m Bd 30 K proteins. On the other hand, several T3seeds had mutant alleles with putative in-frame mutations (Additional file2: Figures S6, S7). The E-type3 haplotype with a 3-nucleotide deletion in theGly m Bd 28 Klocus showed a strong immunoreactive band with the antibody against the Gly m Bd 28 K protein, whereas the expression level of theGly m Bd 28 Kgene was lower than that in Enrei (Fig.5). In this study, the expression level of the targeted loci was examined in only mature seeds of representative mutants and wild-type. Soybean seeds accumulate Gly m Bd 28 K and Gly m Bd 30 K proteins during seed filling [47].Therefore, an investigation of the expression level of the targeted loci in immature seeds might lead to further understanding of accumulation mechanism of mutant proteins.
At least three immunodominant epitopes in Gly m Bd 28 K and five in Gly m Bd 30 K have been identified [48,49,50].In this study, gRNAs were designed against the fourth exon ofGly m Bd 28 Kand first exon ofGly m Bd 30 K(Fig.1). Immunodetection of proteins generated by the in-frame mutations in T3seeds would indicate the presence proteins with preserved epitopes (Additional file2: Figures S6, S7). Analysis of sera of soybean-allergic patients may further clarify the allergenic properties of soybean seeds generated in this study.
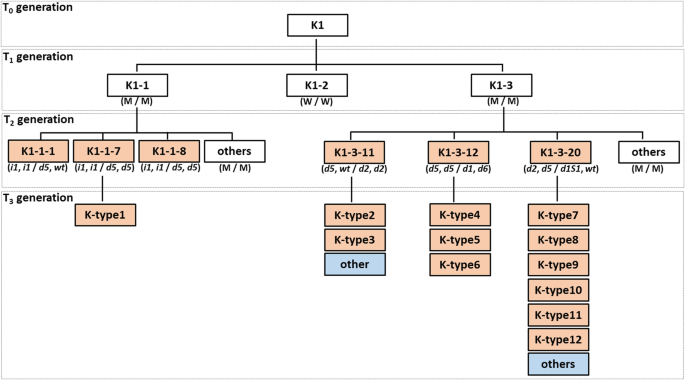
Multiple mutant alleles were detected in the progeny of one T0plant (Fig.7). Three mutant alleles (i1,d2, andd5) in theGly m Bd 28 Klocus and five (d1,d2,d5,d6, andd1s1) mutant alleles in theGly m Bd 30 Klocus were ascertained in theCas9-free T2and T3seeds derived from the K1 T0plant (Fig.7). These mutations appeared after the T2generation, when the distribution of mutant alleles in the targeted loci was validated in the genealogy of the K1 plant and its progeny (Fig.7). Twelve haplotypes (K-type1 to K-type12) were consequently obtained in theCas9-free T3seeds (Fig.7). Previously, we showed that simultaneous site-directed mutagenesis of duplicated loci using a single gRNA resulted in heterozygous and/or chimeric mutations in the targeted loci in most of the T1plants [36].On the other hand, the mutant alleles of multiple targeted loci have been induced in early generations such as T0or T1plants in other studies on soybean site-directed mutagenesis by the CRISPR/Cas9 system [37,51,52].This difference might be explained by different growth and maturity habits of the soybean varieties used. Kariyutaka has early flowering and a short period of vegetative growth [53]; the latter might decrease the chance of the occurrence of mutations in germ cells in the T0generation, however, might produce multiple mutant alleles after the T1generation. Therefore, the site-directed mutagenesis using Kariyutaka might be useful system for obtaining multiple mutant alleles in targeted genes efficiently in a limited number of transgenic soybean plants.
Multiple mutant alleles in the targeted loci in descendants of one T0plant (K1). White boxes, transgenic; light orange boxes,Cas9-free double-mutants; light blue boxes,Cas9-free single mutants in the targeted loci. In parentheses (T0and T1): M, mutant type; W, wild type. Designations of mutations (T3) are as in Table3. In parentheses, the left description of “/” refers to genotypes ofGly m Bd 28 Klocus and the right one to genotypes toGly m Bd 30 Klocus in all transgenic generations
Conclusion
We usedAgrobacterium-mediated transformation and two gRNAs for simultaneous site-directed mutagenesis of two allergenic genes,Gly m Bd 28 KandGly m Bd 30 K, in two Japanese soybean varieties.Cas9-free plants that had mutant alleles of the targeted loci and transgenes excluded by genetic segregation were obtained in the T2or T3generation. Immunoblot analysis revealed that the double-mutants did not accumulate Gly m Bd 28 K or Gly m Bd 30 K protein in mature seeds. Our results showed that simultaneous site-directed mutagenesis by the CRISPR/Cas9 system removed two major allergenic proteins in mature soybean seeds.
Methods
Vector construction
We constructed a gRNA expression vector (pLeg-base) which contained two gRNA expression cassettes. The frame sequence of the gRNA scaffold was derived from the vector pEn-Chimera [54].拟南芥的启动子区域U6-26 [54] and soybean U6–16g [55] were used to control gRNA and gRNA scaffold expression (Fig.1b). The soybean allergenic genesGly m Bd 28 K(Glyma.U020300.1) andGly m Bd 30 K(Glyma.08G116300.1) were the targets for simultaneous site-directed mutagenesis (Fig.1a). Two 20-nucleotide sequences (5′-CTCAGCGAACCGGATATTGG-3′ and 5′-ACCCAAGTAAAGTACCAAGG-3′) identical in each gene were used to design the gRNA sequences with the web-based CRISPR-P 2.0 (http://crispr.hzau.edu.cn/CRISPR2/). The pLeg-base vector was digested with the BbsI or BsaI restriction enzyme (NEB, Ipswich, USA). Oligonucleotides designed to match the gene-specific sequence were annealed to each other to form the gRNA seed sequence, which was ligated into pLeg-base. The CRISPR/Cas9 expression plasmid (pMR284_28K_30K) was constructed by inserting the gRNA expression cassettes of pLeg-base into a Cas9-binary vector (pMR284) harboringCas9and glufosinate resistance gene (Bar) expression cassettes using LR Clonase (Thermo Fisher Scientific, Waltham, USA).
Soybean transformation
The Japanese soybean varieties Enrei (JP 28862) and Kariyutaka (JP 86520) were obtained from Genebank, National Agriculture and Food Research Organization (https://www.gene.affrc.go.jp/index_en.php).Agrobacterium-mediated transformation was performed as described in [28], except that the concentration of glufosinate for selection of transformed cells was decreased from 6 mg/L to 4 mg/L for Enrei.Agrobacterium tumefaciensEHA105窝藏质粒pMR284_28K_30K是我们ed. Transgenic plants were grown in commercial soil (Katakura Chikkarin Co., Tokyo, Japan) at 25 °C in an isolated greenhouse for transgenic plants.
Extraction of genomic DNA and detection of mutations in targeted loci by CAPS analysis
To extract leaf genomic DNA, leaf pieces (approximately 5 mm × 5 mm) were homogenized in 200 μL of extraction buffer [2% CTAB (hexadecyltrimethyl-ammonium bromide), 100 mM Tris-HCl (pH 8.0), 20 mM EDTA (pH 8.0), 1.4 M NaCl, and 0.07% 2-mercaptethanol] in a BioMasher II tube (Nippi, Tokyo, Japan). To extract genomic DNA from mature seeds, a part of cotyledon was powdered and approximately 5 mg of powder was stirred in extraction buffer [10 mM Tris-HCl (pH 8.0), 5 mM EDTA, 0.5% SDS, 0.5% NP40, 0.5% Tween 20, and 80 mg/L proteinase-K (Wako, Osaka, Japan)]. The mixture was incubated at 50 °C for 1 h. DNA extracts were deproteinized with a mixture of phenol, chloroform, and isoamyl alcohol (25:24:1). DNA was precipitated from the supernatant with 2-propanol. The targeted regions in theGly m Bd 28 KandGly m Bd 30 Kloci were amplified by PCR with specific primers (Additional file1:表S3)。The PCR was performed under the following conditions: 30 cycles of 94 °C for 30 s, 54 °C (theGly m Bd 28 K) or 60 °C (theGly m Bd 30 K) for 30 s and 72 °C for 60 s. The amplified products were digested with the DdeI and BsaJI restriction enzymes (NEB), respectively, and separated by electrophoresis in 2.0% agarose gels. The DNA fragments of expected digested-pattern derived from the targeted region carrying mutations and those with no mutations were considered as the mutant type and wild type, respectively. DNA fragments of unexpected size were also regarded as mutant type.
DNA sequencing
The targeted and flanking regions of theGly m Bd 28 KandGly m Bd 30 Kloci were amplified with specific primers (Additional file1:表S3)。The amplified products were cloned into the pGEM-T-Easy vector (Promega, Madison, USA) and sequenced with the Big Dye terminator cycle method using an ABI3100 or ABI3130 Genetic Analyzer (Thermo Fisher Scientific). DNA sequencing analysis was performed by the Instrumental Analysis Division, Graduate School of Agriculture, Hokkaido University.
Selection ofCas9-free plants
To confirm the integration of the Cas9 and gRNA expression module in T1–T3generations, PCR analysis was performed using primers specific for theCas9gene (Additional file1:表S3)。PCR是simultaneou也表现sly amplify endogenous Glyma.01G214600 as a positive control. The PCR was performed under the following conditions: 30 cycles of 94 °C for 30 s, 54 °C for 30 s and 72 °C for 30 s. The existence of theCas9gene were identified by the existence of products amplified by the PCR.
Protein analyses in mature seeds
Soy meal was collected from mature seeds. The extraction of crude protein and protein separation were performed as described in [28].Proteins were separated by SDS-PAGE in a precast 5–12% gradient gel (ATTO, Tokyo, Japan) and transferred onto a PVDF membrane (Hybond-P; GE Healthcare, Little Chalfont, UK). Membranes were blocked with 5% skim milk (Wako) overnight at 4 °C. Recombinant Gly m Bd 30 k was prepared using the baculovirus expression system as described in [5].Using the pET52 vector (Merck-Millipore, Burlington, USA), His10-tagged Gly m Bd 28 K was expressed inEscherichia coliBL21(DE3). After sonication and centrifugation, Gly m Bd 28 K–containing pellets were dissolved in phosphate-buffered saline containing 8 M urea, and Gly m Bd 28 K was purified using a HisTrapFF crude column (GE Healthcare). Antisera were raised in rabbits against the recombinant proteins as described in [56].Immunoreactive bands were detected with the antisera and the ECL Plus Western Blotting system (GE Healthcare).
Expression analysis by semi-quantitative RT-PCR
Total RNA was extracted from mature seeds of mutants, Enrei, and Kariyutaka by the LiCl precipitation procedure [28].Semi-quantitative RT-PCR was conducted in a 20-μL volume using 30 or 38 cycles of 94 °C for 30 s, 57 °C for 30 s, and 72 °C for 10 s. The transcript level of theGly m Bd 28 Kand theGly m Bd 30 Kgene was evaluated relative to that of the18S rRNAgene (XR_003264275).
Genome sequencing
Total DNA was isolated from fresh leaves (1.0–2.0 g) of wild-type and T2plants as described in [41].Genomic DNA libraries were constructed using a TruSeq DNA PCR-Free Library Preparation Kit (Illumina, San Diego, USA).Whole-genome sequencing was conducted on an Illumina HiSeq X platform to obtain 151-nt paired-end reads. Approximately 50× coverage data were obtained for each sample. Unintended remaining foreign DNA was detected as described in [42].
Availability of data and materials
The datasets supporting this study are included within the manuscript and its additional files. The vector developed in this study is available from the corresponding author on reasonable request. The datasets of genomic sequence in Kariyutaka and mutants (K2–1-16 and K4–1-37) have been deposited in the DDBJ Sequence Read Archive under the BioProject Accession PRJDB10633.
Abbreviations
- PVDF:
-
Polyvinylidene difluoride
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
Liu K. Soybean as functional foods and ingredients. New York: Chapman & Hall; 1997.
Utsumi S, Damodaran S, Kinsella JE. Heat-induced interactions between soybean proteins - preferential association of 11S basic subunits and beta-subunits of 7S. J Agric Food Chem. 1984;32:1406–12.
Nagano T, Mori H, Nishinari K. Effect of heating and cooling on the gelation kinetics of 7S globulin from soybeans. J Agric Food Chem. 1994;42:1415–9.
Heppell LM, Sissons JW, Pedersen HE. A comparison of the antigenicity of soya-bean-based infant formulas. Br J Nutr. 1987;58:393–403.
Maruyama N, Sato S, Cabanos C, Tanaka A, Ito K, Ebisawa M. Gly m 5/Gly m 8 fusion component as a potential novel candidate molecular for diagnosing soya bean allergy in Japanese children. Clin Exp Allergy. 2018;48:1726–34.
Ogawa T, Tsuji H, Bando N, Kitamura K, Zhu YL, Hirano H, et al. Identification of the soybean allergenic protein, Gly-m Bd 30k, with the soybean seed 34-kDa oil-body-associated protein. Biosci Biotechnol Biochem. 1993;57:1030–3.
Tsuji H, Bando N, Hiemori M, Yamanishi R, Kimoto M, Nishikawa K, et al. Purification and characterization of soybean allergen Gly m Bd 28K. Biosci Biotechnol Biochem. 1997;61:942–7.
冈萨雷斯R,马球F,萨帕特罗L, Caravaca F, Carreira J. Purification and characterization of major inhalant allergens from soybean hulls. Clin Exp Allergy. 1992;22:748–55.
Codina R, Lockey RF, Fernandez-Caldas E, Rama R. Purification and characterization of a soybean hull allergen responsible for the Barcelona asthma outbreaks. 2. Purification and sequencing of the Gly m 2 allergen. Clin Exp Allergy. 1997;27:424–30.
Rihs HP, Chen ZP, Rueff F, Petersen A, Rozynek P, Heimann H, et al. IgE binding of the recombinant allergen soybean profilin (rGly m 3) is mediated by conformational epitopes. J Allergy Clinic Immun. 1999;104:1293–301.
Kleine-Tebbe J, Wangorsch A, Vogel L, Crowell DN, Haustein UF, Vieths S. Severe oral allergy syndrome and anaphylactic reactions caused by a bet v 1-related PR-10 protein in soybean, SAM22. J Allergy Clinic Immun. 2002;110:797–804.
Ogawa T, Samoto M, Takahashi K. Soybean allergens and hypoallergenic soybean products. J Nutr Sci Vitaminol. 2000;46:271–9.
Thanh VH, Shibasaki K. Major proteins of soybean seeds - straightforward fractionation and their characterization. J Agric Food Chem. 1976;24:1117–21.
Iwabuchi S, Yamauchi F. Electrophoretic analysis of whey proteins present in soybean globulin fractions. J Agric Food Chem. 1987;35:205–9.
Samoto M, Maebuchi M, Miyazaki C, Kugitani H, Kohno M, Hirotsuka M, et al. Abundant proteins associated with lecithin in soy protein isolate. Food Chem. 2007;102:317–22.
Samoto M, Akasaka T, Mori H, Manabe M, Ookura T, Kawamura Y. Simple and efficient procedure for removing the 34kDa allergenic soybean protein,Gly mI, from defatted soy milk. Biosci Biotechnol Biochem. 1994;58:2123–5.
Mori T, Utsumi S, Inaba H, Kitamura K, Harada K. Differences in composition of glycinin among soybean cultivars. J Agric Food Chem. 1981;29:20–3.
Kitamura K, Davies CS, Nielsen NC. Inheritance of alleles for Cgy1and Gy4storage protein genes in soybean. Theor Appl Genet. 1984;68:253–7.
Takahashi K, Banba H, Kikuchi A, Ito M, Nakamura S. An induced mutant line lacking the α-subunit of β-conglycinin in soybean [Glycine max(L.) Merrill]. Breed Sci. 1994;44:65–6.
Hajika M, Takahashi M, Sakai SJ, Igita K. A new genotype of 7 S globulin (beta-conglycinin) detected in wild soybean (Glycine sojaSieb et Zucc). Breed Sci. 1996;46:385–6.
Tsubokura Y, Hajika M, Kanamori H, Xia ZJ, Watanabe S, Kaga A, et al. The β-conglycinin deficiency in wild soybean is associated with the tail-to-tail inverted repeat of the α-subunit genes. Plant Mol Biol. 2012;78(3):301–9.
Hajika M, Takahashi M, Sakai S, Matsunaga R. Dominant inheritance of a trait lacking β-conglycinin detected in a wild soybean line. Breed Sci. 1998;48:383–6.
Takahashi K, Mizuno Y, Yumoto S, Kitamura K, Nakamura S. Inheritance of the α-subunit deficiency of β-conglycinin in soybean (Glycine maxγ射线照射诱导L美林)线。Breed Sci. 1996;46:251–5.
Manjaya JG, Suseelan KN, Gopalakrishna T, Pawar SE, Bapat VA. Radiation induced variability of seed storage proteins in soybeanGlycine max(L.) Merrill. Food Chem. 2007;100:1324–7.
Schmidt MA, Hymowitz T, Herman EM. Breeding and characterization of soybean triple null; a stack of recessive alleles of Kunitz trypsin inhibitor, soybean agglutinin, and P34 allergen nulls. Plant Breed. 2015;134:310–5.
Herman EM, Helm RM, Jung R, Kinney AJ. Genetic modification removes an immunodominant allergen from soybean. Plant Physiol. 2003;132:36–43.
Nishizawa K, Takagi K, Teraishi M, Kita A, Ishimoto M. Application of somatic embryos to rapid and reliable analysis of soybean seed components by RNA interference-mediated gene silencing. Plant Biotechnol. 2010;27:409–20.
Yamada T, Mori Y, Yasue K, Maruyama N, Kitamura K, Abe J. Knockdown of the 7S globulin subunits shifts distribution of nitrogen sources to the residual protein fraction in transgenic soybean seeds. Plant Cell Rep. 2014;33:1963–76..
Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:82.
Li JF, Norville JE, Aach J, McCormack M, Zhang DD, Bush J, et al. Multiplex and homologous recombination-mediated genome editing inArabidopsisandNicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol. 2013;31:688–91.
Nekrasov V, Staskawicz B, Weigel D, Jones JDG, Kamoun S. Targeted mutagenesis in the model plantNicotiana benthamianausing Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:691–3.
Shan QW, Wang YP, Li J, Zhang Y, Chen KL, Liang Z, et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol. 2013;31:686–8.
Li ZS, Liu ZB, Xing AQ, Moon BP, Koellhoffer JP, Huang LX, et al. Cas9-guide RNA directed genome editing in soybean. Plant Physiol. 2015;169:960–70.
Cai Y, Chen L, Liu X, Guo C, Sun S, Wu C, et al. CRISPR/Cas9-mediated targeted mutagenesis ofGmFT2adelays flowering time in soybean. Plant Biotechnol J. 2018;16:176–85.
Curtin SJ, Xiong Y, Michno J-M, Campbell BW, Stec AO, Čermák T, et al. CRISPR/Cas9 and TALENs generate heritable mutations for genes involved in small RNA processing ofGlycine maxandMedicago truncatula. Plant Biotechnol J. 2018;16:1125–37.
Kanazashi Y, Hirose A, Takahashi I, Mikami M, Endo M, Hirose S, et al. Simultaneous site-directed mutagenesis of duplicated loci in soybean using a single guide RNA. Plant Cell Rep. 2018;37:553–63.
Do PT, Nguyen CX, Bui HT, Tran LTN, Stacey G, Gillman JD, et al. Demonstration of highly efficient dual gRNA CRISPR/Cas9 editing of the homeologousGmFAD2-1AandGmFAD2-1Bgenes to yield a high oleic, low linoleic and α-linolenic acid phenotype in soybean. BMC Plant Biol. 2019;19:311.
Nakamura T, Utsumi S, Kitamura K, Harada K, Mori T. Cultivar differences in gelling characteristics of soybean glycinin. J Agric Food Chem. 1984;32:647–51.
Tezuka M, Taira H, Igarashi Y, Yagasaki K, Ono T. Properties of tofus and soy milk prepared from soybeans having different subunits of glycinin. J Agric Food Chem. 2000;48:1111–7.
Maruyama N, Park K, Motoyama S, Choi S-K, Yagasaki K, Ishimoto M, et al. Structure–physicochemical function relationships of soybean glycinin at subunit levels assessed by using mutant lines. J Agric Food Chem. 2004;52:8197–201.
Yamada T, Watanabe S, Arai M, Harada K, Kitamura K. Cotyledonary node pre-wounding with a micro-brush increased frequency of agrobacterium-mediated transformation in soybean. Plant Biotechnol. 2010;27:217–20.
Itoh T, Onuki R, Tsuda M, Oshima M, Endo M, Sakai H, et al. Foreign DNA detection by high-throughput sequencing to regulate genome-editing agricultural products. Sci Rep. 2020;10:4914.
Ogawa T, Bando N, Tsuji H, Okajima H, Nishikawa K, Sasaoka K. Investigation of the IgE-binding proteins in soybeans by immunoblotting with the sera of the soybean-sensitive patients with atopic-dermatitis. J Nutr Sci Vitaminol. 1991;37:555–65.
Samoto M, Fukuda Y, Takahashi K, Tabuchi K, Hiemori M, Tsuji H, et al. Substantially complete removal of three major allergenic soybean proteins (Gly m Bd 30K, Gly m Bd 28K, and the α-subunit of conglycinin) from soy protein by using a mutant soybean, Tohoku 124. Biosci Biotechnol Biochem. 1997;61:2148–50.
Joseph LM, Hymowitz T, Schmidt MA, Herman EM. Evaluation ofGlycinegermplasm for nulls of the immunodominant allergen P34/Gly m Bd 30k. Crop Sci. 2006;46:1755–63.
Kirchner TW, Niehaus M, Debener T, Schenk MK, Herde M. Efficient generation of mutations mediated by CRISPR/Cas9 in the hairy root transformation system ofBrassica carinata. PLoS One. 2017;12:e0185429.
Wu YM, Guan RX, Liu ZX, Li RZ, Chang RZ, Qiu LJ. Synthesis and degradation of the major allergens in developing and germinating soybean seed. J Integr Plant Biol. 2012;54:4–14.
Helm RM, Cockrell G, Herman E, Burks AW, Sampson HA, Bannon GA. Cellular and molecular characterization of a major soybean allergen. Int Arch Allergy Immunol. 1998;117:29–37.
Helm RM, Cockrell G, Connaughton C, West CM, Herman E, Sampson HA, et al. Mutational analysis of the IgE-binding epitopes of P34/Gly m Bd 30K. J Allergy Clin Immunol. 2000;105:378–84.
Xi J, Yan HL. Epitope mapping and identification of amino acids critical for mouse IgG-binding to linear epitopes on Gly m Bd 28K. Biosci Biotechnol Biochem. 2016;80:1973–9.
Cai YP, Wang LW, Chen L, Wu TT, Liu LP, Sun S, et al. Mutagenesis ofGmFT2aandGmFT5amediated by CRISPR/Cas9 contributes for expanding the regional adaptability of soybean. Plant Biotechnol J. 2020;18:298–309.
Bao AL, Chen HF, Chen LM, Chen SL, Hao QN, Guo W, et al. CRISPR/Cas9-mediated targeted mutagenesis ofGmSPL9genes alters plant architecture in soybean. BMC Plant Biol. 2019;19:131.
Tsubokura Y, Watanabe S, Xia Z, Kanamori H, Yamagata H, Kaga A, et al. Natural variation in the genes responsible for maturity lociE1,E2,E3andE4in soybean. Ann Bot. 2014;113:429–41.
Fauser F, Schiml S, Puchta H. Both CRISPR/Cas-based nucleases and nickases can be used efficiently for genome engineering in Arabidopsis thaliana. Plant J. 2014;79:348–59.
Du HY, Zeng XR, Zhao M, Cui XP, Wang Q, Yang H, et al. Efficient targeted mutagenesis in soybean by TALENs and CRISPR/Cas9. J Biotechnol. 2016;217:90–7.
Nishizawa K, Maruyama N, Satoh R, Fuchikami Y, Higasa T, Utsumi SA. C-terminal sequence of soybean β-conglycinin α' subunit acts as a vacuolar sorting determinant in seed cells. Plant J. 2003;34:647–59.
Acknowledgements
We thank Professor Holger Puchta (University of Karlsruhe) for permission to use plasmid DNA of pEn-Chimera, Dr. Mily Ron and Professor Anne Britt (UC Davis) for kindly supply of pMR284, and M. Suzuki, S. Noguchi, Y. Kitsui, and R. Onuki for general technical assistance. We thank the DASH (Development and Assessment of Sustainable Humanosphere) system for supporting the experiment.
Funding
This work was supported by the Cabinet Office, Government of Japan [the Cross-ministerial Strategic Innovation Promotion Program (SIP)] to TY. The funding agency was not involved in the designing of the study and collection, analysis and interpretation of the data, and in the writing of the manuscript.
Author information
Affiliations
Contributions
SS, JA, and TY: conceived and designed the experiments; AH, YK, MM, ME, and SH: constructed vectors; SS, AH, and YK: performed site-directed mutagenesis; SS, KA, MH, and NM: analyzed mutations in transgenic soybean; TI: analyzed unintended remaining foreign DNA; SS, JA, and TY: contributed to the writing the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Induction of mutations in the targeted loci and the integration of theCas9gene in representative T1plants from the transformation of Enrei.Table S2.Induction of mutations in the targeted loci and the integration of theCas9gene in representative T1plants from the transformation of Kariyutaka.Table S3.Primer sequences used for vector construction, confirmation of transgenes, and CAPS, semi-quantitative RT-PCR, and sequencing analyses.
Additional file 2: Figure S1.
Confirmation of mutagenesis of targeted loci in representative Kariuytaka-T1plants by CAPS analysis.Figure S2.突变的检测Gly m Bd 28KandGly m Bd 30Kloci in representative Enrei-T2seeds by CAPS analysis.Figure S3.突变的检测Gly m Bd 28KandGly m Bd 30Kloci in representative Kariyutka-T2seeds by CAPS analysis.Figure S4.Detection of the integration of theCas9gene in representative T2seeds by PCR analysis.Figure S5.Mutational spectra of the targeted loci in double-mutant T3seeds.Figure S6.Alignment of predicted amino acid sequences of theGly m Bd 28Klocus in double mutants.Figure S7.Alignment of predicted amino acid sequences of theGly m Bd 30Klocus in double mutants.Figure S8.Full-length gel electrophoresis and immunoblot of the crude protein of representative double-mutant T3and wild-type mature seeds.Figure S9.Primer sites used for semi-quantitative RT-PCR analysis of theGly m Bd 30Kand theGly m Bd 30Kloci.Figure S10.Morphological characteristics of representative double-mutant (T2) and control Kariyutaka plants.Figure S11.Morphological characteristics of representative double-mutant (T3) and control Kariyutaka seeds.
Rights and permissions
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sugano, S., Hirose, A., Kanazashi, Y.et al.Simultaneous induction of mutant alleles of two allergenic genes in soybean by using site-directed mutagenesis.BMC Plant Biol20,513 (2020). https://doi.org/10.1186/s12870-020-02708-6
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/s12870-020-02708-6
Keywords
- CRISPR/Cas9
- Glycine max
- Gly m Bd 28 K
- Gly m Bd 30 K
- Hypoallergenic soybean