- Research article
- Open Access
- Published:
Repair of sub-lethal freezing damage in leaves ofArabidopsis thaliana
BMC Plant Biologyvolume20, Article number:35(2020)
Abstract
Background
的detrimental effects of global climate change direct more attention to the survival and productivity of plants during periods of highly fluctuating temperatures. In particular in temperate climates in spring, temperatures can vary between above-zero and freezing temperatures, even during a single day. Freeze-thaw cycles cause cell membrane lesions that can lead to tissue damage and plant death. Whereas the processes of cold acclimation and freeze-thaw injury are well documented, not much is known about the recovery of plants after a freezing event. We therefore addressed the following questions: i. how does the severity of freezing damage influence repair; ii. how are respiration and content of selected metabolites influenced during the repair process; and iii. how do transcript levels of selected genes respond during repair?
Results
We have investigated the recovery from freezing to sub-lethal temperatures in leaves of non-acclimated and cold acclimatedArabidopsis thalianaplants over a period of 6 days. Fast membrane repair and recovery of photosynthesis were observed 1 day after recovery (1D-REC) and continued until 6D-REC. A substantial increase in respiration accompanied the repair process. In parallel, concentrations of sugars and proline, acting as compatible solutes during freezing, remained unchanged or declined, implicating these compounds as carbon and nitrogen sources during recovery. Similarly, cold-responsive genes were mainly down regulated during recovery of cold acclimated leaves. In contrast, genes involved in cell wall remodeling and ROS scavenging were induced during recovery. Interestingly, also the expression of genes encoding regulatory proteins, such as 14–3-3 proteins, was increased suggesting their role as regulators of repair processes.
Conclusions
Recovery from sub-lethal freezing comprised membrane repair, restored photosynthesis and increased respiration rates. The process was accompanied by transcriptional changes including genes encoding regulatory proteins redirecting the previous cold response to repair processes, e.g. to cell wall remodeling, maintenance of the cellular proteome and to ROS scavenging. Understanding of processes involved in repair of freeze-thaw injury increases our knowledge on plant survival in changing climates with highly fluctuating temperatures.
Background
Effects of global climate change, such as milder winters and higher temperature fluctuations in spring in the Northern parts of the world strongly impact the survival of plants due to late-season cold spells. Cold acclimation, which increases freezing tolerance of plants during exposure to low, but non-freezing temperatures, improves winter survival [1]。Most plants native to temperate and boreal climates undergo an annual cycle of acclimation and deacclimation to increase freezing tolerance in fall and reduce tolerance in spring [2,3]。
Highly fluctuating temperatures result in frequent freeze-thaw cycles, causing membrane lesions by membrane contraction and expansion [4,5], affecting plant productivity and resulting in economic losses for agriculture [6]。Extensive frost damage in spring after a warm spell has been documented repeatedly [7,8,9,10]。
Since tissues are injured by both freeze-induced dehydration and rehydration during thawing [1,11], the term “freeze-thaw stress tolerance” (FTST) was defined to describe the ability of a plant to survive a freeze-thaw cycle and to recover from non-lethal lesions after thawing [12,13]。Whereas freeze-thaw injury has been investigated in detail, little attention was paid to the ability of injured tissue to recover from freeze-thaw stress, although this is critical for FTST [14,15,16]。Freeze-thaw injury is mainly caused by extracellular ice formation provoking freeze-dehydration which results in membrane destabilization [5]。Though the plasma membrane is considered as the primary site of injury [17], there is also extensive damage to the chloroplast envelope and thylakoid membranes [18] leading to decreased photosynthetic activity [19]。In addition, some chloroplast stromal enzymes such as Rubisco are inactivated during freezing [20]。
Physiological and molecular processes involved in post-thaw recovery from non-lethal lesions, critical for frost survival, have only scarcely been investigated. During post-thaw recovery of injured tissues, membrane damage is reversed and turgor regained, e.g. during full recovery of onion cells [21]。Proteomic studies in onion showed that proteins responsible for cell repair, affecting reactive oxygen species (ROS) scavenging, removal and assembly of denatured proteins, membrane and cell wall stabilization and restoration of the cellular ionic environment accumulated during post-thaw recovery [13]。Another study in spinach supported these findings, with post-thaw recovery accompanied by a reduction in ion leakage, recovery of photosystem II efficiency, activation of antioxidant enzymes and dissipation of ROS [22]。
Compatible solutes such as sugars or proline that are known to stabilize membranes during freezing accumulate during cold acclimation and decrease during deacclimation [23,24]。的y might also play a role for the level of freeze-thaw damage and post-thaw recovery. They furthermore increase the osmotic potential of the cells, stabilize proteins during freezing [2,25] and act as ROS scavengers [26]。Metabolic changes inAvena sativacrowns recovering from freezing over 14 days included increased amounts of amino acids and decreased amounts of sucrose, fructose and TCA cycle intermediates [27]。
在本文中,我们量化修复公关ocesses after a freeze-thaw cycle at the plasma membrane using an electrolyte leakage assay and at the chloroplast using chlorophyll fluorescence measurements in leaves of non-acclimated and cold acclimated Arabidopsis plants. In addition, we determined respiratory activity and investigated pool sizes of proline, glucose, fructose, sucrose and raffinose as indicators of metabolic activity during recovery. The expression of genes orthologous to genes encoding proteins previously identified as significantly altered in abundance during recovery in onion [13] was investigated by qRT-PCR. The results provide new insights into the repair process after sub-lethal freeze-thaw injury in Arabidopsis and identify transcriptional regulation to be important for post-thaw recovery.
Results
Sub-lethal freezing injury is quickly repaired after thawing
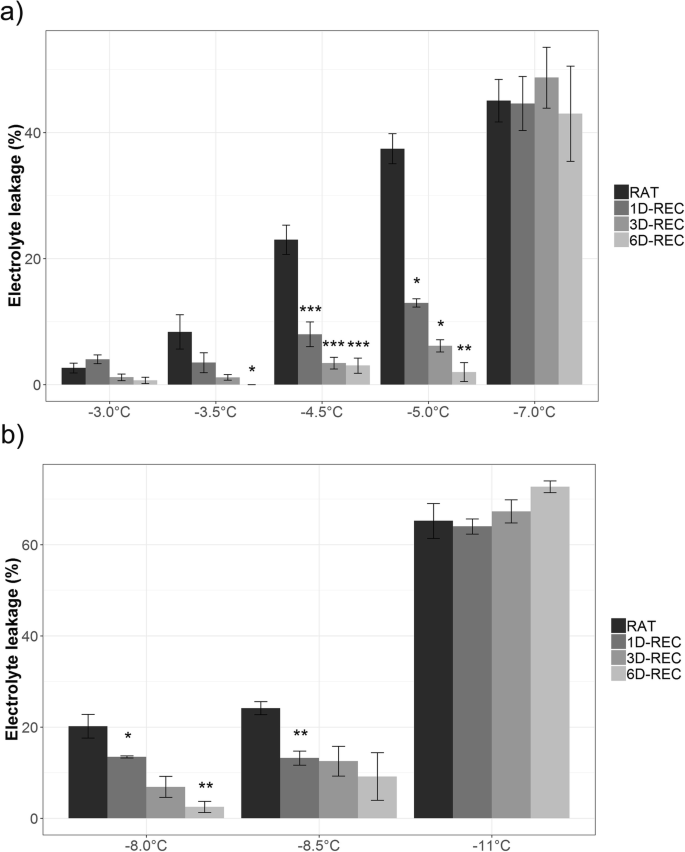
Freeze-thaw injury and post-thaw recovery were analyzed for fully developed leaves by electrolyte leakage measurements after freezing to five different temperatures (Table1) for non-acclimated (NA) and three temperatures for cold-acclimated (ACC)Arabidopsis thalianaCol-0 plants. Freezing temperatures slightly higher (warmer) than the previously reported LT50values of − 5.34 °C NA and − 9.68 °C ACC [28] were chosen to generate non-lethal lesions in contrast to irreversible injury after freezing to temperatures below (colder than) the respective LT50.电解质渗漏测定样品对吗t after thawing at 4 °C (RAT) or at 1D-REC, 3D-REC and 6D-REC at 4 °C. Recovery at 20 °C caused unreliable electrolyte leakage values due to bacterial growth (not shown) and was therefore not further investigated. At mild freezing temperatures, such as − 3 °C, electrolyte leakage at RAT was very low (2.65%), but increased gradually with decreasing temperatures to 45.10% when non-acclimated leaves were frozen to − 7 °C (Fig.1a).
Relative electrolyte leakage values of Arabidopsis leaves frozen to different temperatures right after thawing (RAT) or after recovery for 1 day (1D-REC), 3 days (3D-REC) or 6 days (6D-REC) at 4 °C foranon-acclimated plants andbcold acclimated plants. Averages of four replicates are shown with standard errors. Significance levels between values measured at RAT and at the subsequent time points determined by Student’s t-test are indicated as: ***,p < 0.001, **,p < 0.01, *,p < 0.05
Already after 1D-REC electrolyte leakage was significantly decreased and it decreased further over 6D-REC even in leaves frozen to − 5 °C. No recovery from freeze-thaw injury was observed after freezing to − 7 °C. A similar pattern was observed for leaves of cold acclimated plants (Fig.1b) with increasing injury at RAT conditions following exposure to decreasing freezing temperatures and a recovery process over 6D-REC when the freezing temperature was above the previously reported LT50ACC. At a freezing temperature below the LT50ACC (− 11 °C), no recovery was observed and electrolyte leakage values actually increased from 65.2% at RAT to 72.7% 6D-REC.
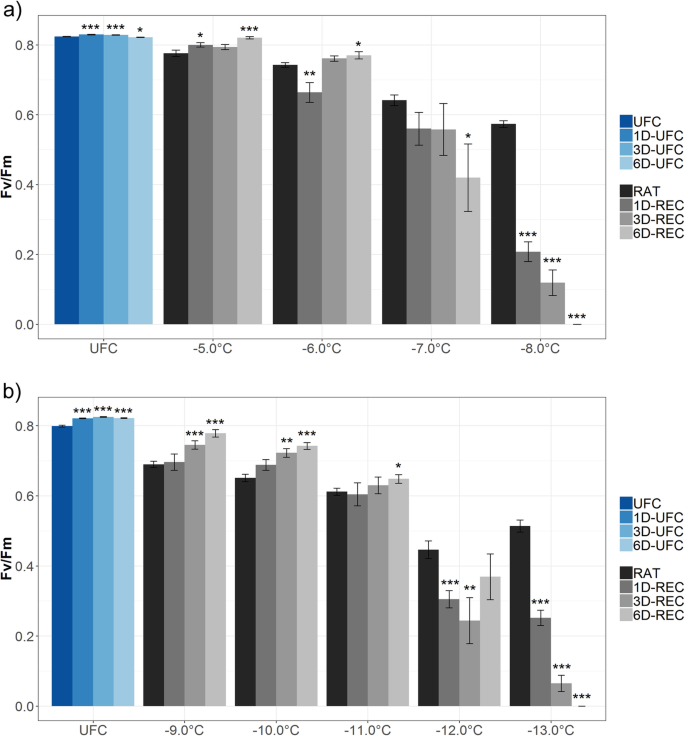
Chlorophyll fluorescence imaging measurements were performed with leaves from non-acclimated and cold-acclimated plants to reveal potential repair of freezing damage in chloroplasts. For these experiments, lower freezing temperatures than for the electrolyte leakage measurements were used (− 5 °C to − 8 °C for non-acclimated and − 9 °C to − 13 °C for acclimated leaves). Chlorophyll fluorescence imaging consistently yields lower (more negative) LT50values than electrolyte leakage assays [19] arguably because the preincubation in water before the conductivity measurements causes secondary damage to the leaves, resulting in greater ion leakage and thus a slight underestimation of LT50.Decreasing Fv/Fm ratios represent a decreasing maximum quantum yield efficiency of PSII and therefore reflect damage to the thylakoid membranes. When non-acclimated leaves were frozen to decreasing temperatures, the Fv/Fm ratio at RAT decreased from 0.78 (at − 5 °C) to 0.57 (at − 8 °C) compared to 0.82 in the unfrozen control (UFC) leaves (Fig.2a). Fv/Fm ratio at 6D-REC increased to values similar to control conditions when the leaves were frozen to − 5 °C. Also, leaves frozen to − 6 °C were able to recover after an initial additional drop of the Fv/Fm ratio at 1D-REC resulting in values above the RAT at 6D-REC but slightly below the respective control. At lower freezing temperatures, the initial injury was not reversed and the Fv/Fm ratio decreased further to 0.42 (− 7 °C) or even to 0 (− 8 °C).
Maximum quantum yield efficiency of PSII (Fv/Fm) in unfrozen Arabidopsis leaves (UFC, 1D-UFC, 3D-UFC, 6D-UFC) and in leaves frozen to different temperatures right after thawing (RAT) or after recovery for 1 day (1D-REC), 3 days (3D-REC) or 6 days (6D-REC) at 4 °C foranon-acclimated plants andbcold acclimated plants. Control samples were incubated at 4 °C for the respective times. Three independent experiments were carried out for each temperature except for − 5 °C, − 7 °C, − 11 °C and − 12 °C with only one and − 13 °C with two experiments. Data are means of 10 to 15 biological replicates per experiment with standard errors. Significance levels between values measured at UFC/RAT and at the subsequent time points determined by Student’s t-test are indicated as: ***,p < 0.001, **,p < 0.01, *,p < 0.05
Leaves from cold acclimated plants showed a similar decline in the Fv/Fm ratios at RAT conditions with decreasing freezing temperatures (Fig.2b). UFC leaves showed a maximum quantum yield of 0.80 to 0.82. Leaves frozen to − 9 °C or − 10 °C displayed a continuous increase in Fv/Fm during 6D-REC from 0.69 to 0.78 or from 0.65 to 0.74, respectively. Leaf samples frozen to − 11 °C still showed a small increase in the Fv/Fm ratio at 6D-REC. Samples exposed to lower freezing temperatures showed a decrease of the Fv/Fm values over time which reached zero at 6D-REC after freezing to − 13 °C.
Respiration rate is increased during recovery
Oxygen consumption is highly influenced by the temperature at which respiration rates are measured [29]。Since the recovery process took place at 4 °C, we measured respiration rates during recovery at that temperature (Fig.3a, c). In addition, measurements were also performed at 21 °C (Fig.3b, d), a temperature more generally used for such measurements. Samples from non-acclimated and cold acclimated plants were frozen to either − 6 °C, − 7 °C and − 8 °C, or to − 8 °C, − 9 °C and − 12 °C, respectively. Higher respiration rates were measured in all samples processed at 21 °C compared to 4 °C, with an average over all samples of non-acclimated plants of 238 nmol O2min− 1 mg− 1FW versus 134 nmol O2min− 1 mg− 1FW at 4 °C and over all respective samples of cold acclimated plants of 370 nmol O2min− 1 mg− 1FW versus 131 nmol O2min− 1 mg− 1FW at 4 °C.
Respiration rates measured as oxygen consumption in unfrozen Arabidopsis leaves (UFC, 1D-UFC, 3D-UFC, 6D-UFC) and in leaves frozen to different temperatures right after thawing (RAT) or after recovery for 1 day (1D-REC), 3 days (3D-REC) or 6 days (6D-REC) at 4 °C fora,bnon-acclimated plants andc,dcold acclimated plants.a,cRespiration was measured at the recovery temperature (4 °C);b,drespiration was measured at room temperature (21 °C). Data are the means of five replicates with two leaf discs each with standard errors. Significance levels between values measured at UFC/RAT and at the subsequent time points determined by Student’s t-test are indicated as: ***,p < 0.001, **,p < 0.01, *,p < 0.05
Measurements at 4 °C revealed increased respiration rates during the recovery time in UFC samples, in particular for non-acclimated leaves (Fig.3a). In addition, when non-acclimated samples were frozen to − 6 °C, or acclimated samples to − 8 °C, respiration rates increased 4.4-fold (Fig.3a) or 2.2-fold (Fig.3c) during recovery at 3D-REC compared to RAT. There were no comparatively large increases evident when leaves were frozen to lower temperatures. When measurements were performed at 21 °C, a strong increase in respiration rates was recorded at all recovery points for non-acclimated leaves frozen to − 6 °C or − 7 °C. No consistent changes in respiration rates were observed in acclimated leaves measured at 21 °C through the recovery process (Fig.3d).
Sugar and proline content after freeze-thaw injury and during recovery
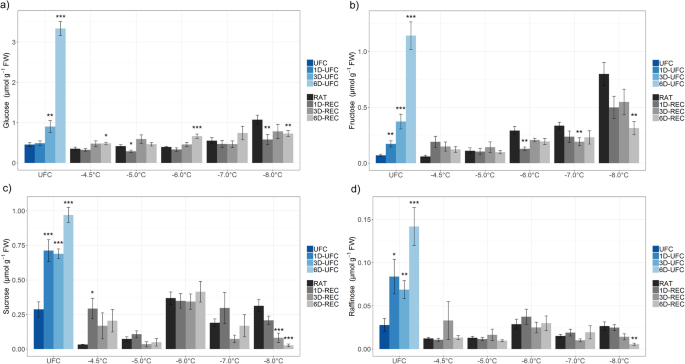
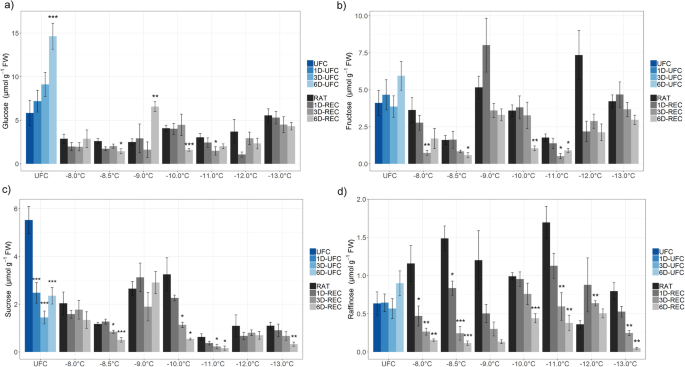
Glucose, fructose, sucrose, and raffinose (Figs.4and5) and the amino acid proline (Fig.6) were measured in leaf samples frozen to temperatures from − 4.5 °C to − 8.0 °C for non-acclimated and from − 8.0 °C to − 13.0 °C for cold acclimated plants. UFC samples from non-acclimated plants showed strong 7.3, 16.4, 3.4 and 5.1 fold increases for glucose, fructose, sucrose and raffinose, respectively, at 6D-UFC compared to UFC conditions (Fig.4) caused by the experimental design with recovery taking place at 4 °C. Proline content, on the other hand, showed no significant difference between UFC and 6D-UFC in the same samples (Fig.6a). Cold acclimation for 7 d resulted in a massive accumulation of all sugars (Fig.5) and proline (Fig.6), in agreement with many earlier reports [2,30]。In cold acclimated UFC leaves, changes were more variable than in non-acclimated leaves, with a significant increase in glucose, a significant decrease in sucrose, and no significant changes in fructose, raffinose and proline content between UFC and 6D-UFC (Figs.5and6b).
在解冻的拟南芥叶糖含量(终极格斗冠军赛,1D-UFC, 3D-UFC, 6D-UFC) and in leaves frozen to different temperatures right after thawing (RAT) or after recovery for 1 day (1D-REC), 3 days (3D-REC) or 6 days (6D-REC) at 4 °C for non-acclimated plants.aglucose,bfructose,csucrosedraffinose. Please note the different scales of the y-axes. Data are the mean with standard errors for five replicates from one experiment except for the non-acclimated UFC and non-acclimated samples at − 6 °C and − 8 °C with three experiments measured. Significance levels between values measured at UFC/RAT and at the subsequent time points determined by Student’s t-test are indicated as: ***,p < 0.001, **,p < 0.01, *,p < 0.05
在解冻的拟南芥叶糖含量(终极格斗冠军赛,1D-UFC, 3D-UFC, 6D-UFC) and in leaves frozen to different temperatures right after thawing (RAT) or after recovery for 1 day (1D-REC), 3 days (3D-REC) or 6 days (6D-REC) at 4 °C for cold acclimated plants.aglucose,bfructose,csucrosedraffinose. Please note the different scaling of the y-axes. Data are the mean with standard errors for five replicates from one experiment. Significance levels between values measured at UFC/RAT and at the subsequent time points determined by Student’s t-test are indicated as: ***,p < 0.001, **,p < 0.01, *,p < 0.05
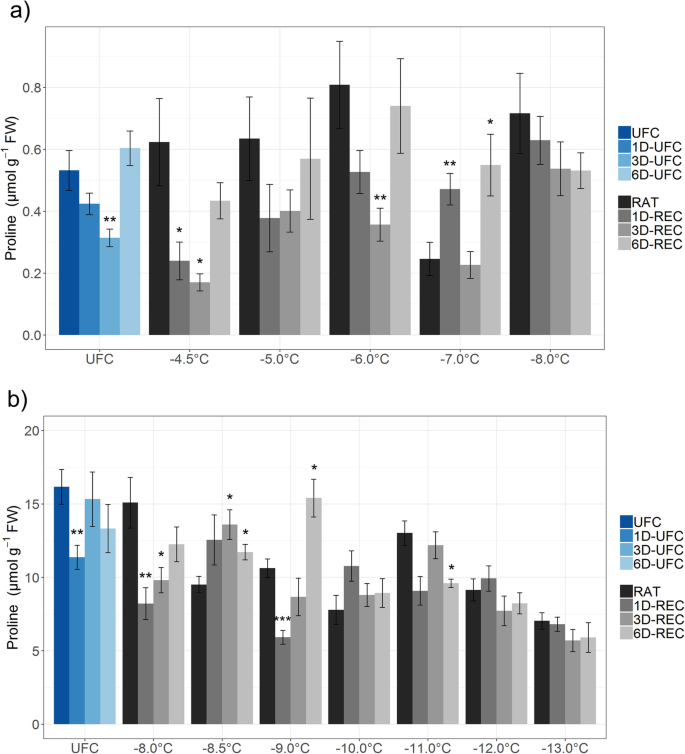
Proline content in unfrozen Arabidopsis leaves (UFC, 1D-UFC, 3D-UFC, 6D-UFC) and in leaves frozen to different temperatures right after thawing (RAT) or after recovery for 1 day (1D-REC), 3 days (3D-REC) or 6 days (6D-REC) at 4 °C foranon-acclimated plants andbcold acclimated plants. Please note the different scales of the y-axes. Shown is the mean with standard errors for five replicates from one experiment except for the non-acclimated UFC and non-acclimated samples at − 6 °C and − 8 °C with three experiments measured. Significance levels between values measured at UFC/RAT and at the subsequent time points determined by Student’s t-test are indicated as: ***,p < 0.001, **,p < 0.01, *,p < 0.05
When leaves from non-acclimated plants were subjected to a freeze-thaw cycle, the levels of glucose (only at − 8 °C) and fructose (at − 6 °C, − 7 °C and − 8 °C) at RAT increased compared to the corresponding value under control conditions. During recovery, no consistent further increases were observed. In fact, at − 8 °C (i.e. below the LT50) sugar levels significantly decreased over time (Fig.4). In leaves from cold acclimated plants frozen to temperatures from − 8 °C to − 13 °C, glucose, and sucrose levels were mostly lower than under control conditions, while fructose levels were similar. Only raffinose content was consistently higher in frozen-thawed leaves at RAT than in the UFC. Recovery had no consistent influence on glucose levels at any freezing temperature, while fructose, sucrose and raffinose levels decreased at most freezing temperatures and recovery time points (Fig.5). In particular raffinose decreased strongly during recovery.
In non-acclimated leaves proline levels decreased at 1D-UFC and 3D-UFC and at 1D-REC and 3D-REC in samples frozen to − 4.5 °C, − 5.0 °C and − 6 °C, although the decreases were not significant at all temperatures and time points (Fig.6a). Quite strikingly, proline increased again at 6D-REC and returned to similar levels as at RAT. In leaves frozen to − 8 °C, proline content showed a decreasing tendency over time, that was, however, not significant. In leaves from cold acclimated plants, proline content decreased gradually at RAT with decreasing freezing temperatures and reached 43% of the control value at − 13 °C (Fig.6b). The pattern of changes in proline during recovery was similar to non-acclimated conditions with an initial decrease and a later increase under control conditions and after freezing to − 8.0 °C and − 9.0 °C. When the leaves were frozen to temperatures between − 10 °C and − 13 °C proline levels did not show consistent changes during 6D-REC.
Expression of genes encoding proteins related to recovery from freeze-thaw injury
Genes related to the recovery process after a freeze-thaw cycle were selected from a proteomic study in onion [13]。Orthologs of 41 onion genes were identified in Arabidopsis and included genes encoding 12 proteins involved in cell wall remodelling, proteins involved in ion and water homeostasis (e.g. three aquaporins), and in ROS scavenging (e.g. four glutathione S-transferases) (Additional file1: Table S1). In addition, we included five cold-responsive genes (COR6.6, COR15A, COR47, COR78, Galactinol synthase 3 - GOLS3) that we have used previously to characterize transcriptional responses of Arabidopsis to cold acclimation [28] and deacclimation [23]。Furthermore some related genes to the ones identified from the onion study, e.g. genes encoding 14–3-3 proteins (GRF5–8) were included. When the expression level of a specific gene was very low at all conditions this gene was excluded from the analysis (e.g. annexin 7 – ANNAT7). cDNAs of leaves from non-acclimated plants frozen to − 5 °C, − 6 °C, − 7 °C, and − 8 °C, and from cold acclimated plants frozen to − 9 °C, − 10 °C, − 11 °C, − 12 °C, and − 13 °C from all recovery time points (RAT, 1D-REC, 3D-REC and 6D-REC) and the respective UFCs were analyzed by qRT-PCR (Additional file3:表S3)。
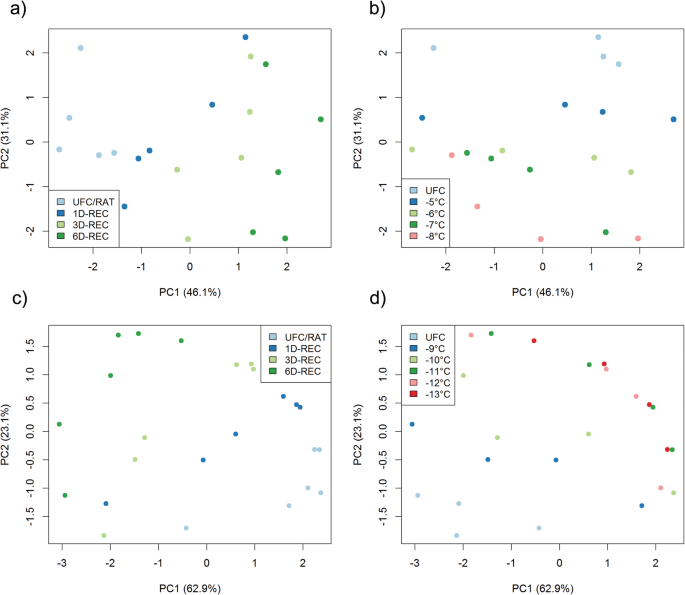
A principal component analysis (PCA) of all generated data showed that Principal Component 2 (PC2) separated samples from non-acclimated and cold acclimated plants, explaining 31% of the variance in the data set (Additional file4: Figure S1). To increase resolution among data generated from leaves frozen to different temperatures and recovered for various durations, we performed PCAs separately with the datasets generated from non-acclimated and cold acclimated plants (Fig.7). Samples were colour coded either for recovery duration (Fig.7a, c) or temperature (Fig.7b, d). PC1 clearly separated all different recovery times from each other under both non-acclimated and cold acclimated conditions and explained 46.1% or 62.9% of the total variance in the datasets, respectively (Fig.7a, c). Samples at the RAT timepoint were separated consecutively further from samples after 1D-REC, 3D-REC and 6D-REC. PC2 separated the samples according to the treatment temperatures and explained 31.1 and 23.1% of the variance under non-acclimated and cold acclimated conditions, respectively (Fig.7b, d). Under non-acclimated conditions, samples were consecutively grouped according to the different treatment temperatures, while under acclimated conditions UFC samples and samples frozen to − 9 °C and − 10 °C were clearly separated from each other. Samples frozen to − 11 °C, − 12 °C and − 13 °C clustered together, but were separated from samples frozen to milder temperatures.
Probabilistic Principal Component Analysis (PCA) using expression data of 41 genes measured by qRT-PCR in unfrozen Arabidopsis leaves (UFC) and in leaves frozen to different temperatures right after thawing (RAT) or after recovery for 1 day (1D-REC), 3 days (3D-REC) or 6 days (6D-REC) at 4 °C fora,bnon-acclimated plants andc,dcold acclimated plants. Samples are color coded according to days after recovery (a,c) or freezing temperature (b,d)
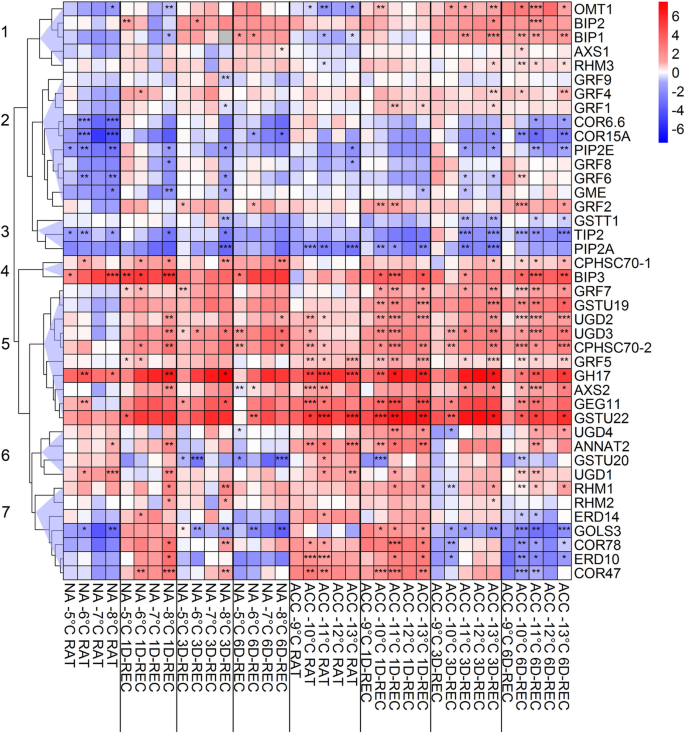
Figure8shows a hierarchical cluster analysis of the changes in transcript abundance (log2在复苏后冻结dif褶皱变化)ferent temperatures relative to the respective control condition. The 41 genes could be grouped into four larger clusters containing five to ten genes (clusters 1, 2, 5, 7) and three smaller clusters containing two to four genes (clusters 3, 4, 6).
Hierarchical cluster analysis (HCA) of the changes in relative transcript abundance of 41 genes in Arabidopsis leaves frozen to different temperatures right after thawing (RAT) or after recovery for 1 day (1D-REC), 3 days (3D-REC) or 6 days (6D-REC) at 4 °C for non-acclimated plants (left part) and cold acclimated plants (right part). The log2FC in transcript abundance at the different conditions relative to the respective control is color coded with blue indicating reduced and red increased transcript abundance. Seven main clusters are represented as blue triangles on the left. Significance levels of the relative expression to the respective temperature control (2-∆Ct) are indicated as: ***,p < 0.001, **,p < 0.01, *,p < 0.05
Cluster 1contains five genes encoding two proteins of the HSP70 family (luminal binding protein 1 and 2, BIP1 and BIP2), a flavonol-3-O-methyltransferase (OMT) and two cell wall related proteins (UDP-D-xylose synthase 1 (AXS1) and rhamnose biosynthetic enzyme 3 (RHM3)). The genes in this cluster were upregulated during recovery with the highest upregulation at 3D-REC and 6D-REC. At RAT conditions, these genes were slightly downregulated in samples of non-acclimated and in selected samples of cold acclimated plants.
Genes incluster 2were mostly downregulated or not significantly changed in expression. Only three genes encoding 14–3-3 like proteins (GRF1, 2, 4) showed significant upregulation at some time points/temperatures. In non-acclimated samples, the expression of almost all genes of cluster 2 was decreased at RAT with significant changes forCOR6.6,COR15A,PIP2E,GRF6andGME(GDP-mannose 3,5-epimerase). After 1D-REC, 3D-REC or 6D-REC the expression of most of these genes increased slightly to levels of the control in leaves frozen to − 5 °C, − 6 °C or − 7 °C, whereas there was still a significant decrease in the expression ofPIP2E,GRF6, GRF8andGMEin samples frozen to − 8 °C. A similar pattern was observed in leaves of acclimated plants with the exception that there were almost no significant changes at RAT and a significant decrease ofCOR6.6,COR15AandPIP2Eat 6D-REC.
Cluster 3comprises three genes encoding glutathione S-transferase (GST) THETA1 (GSTT1), the aquaporins tonoplast intrinsic protein 2 (TIP2) and plasma membrane intrinsic protein 2A (PIP2A). All three genes showed decreased expression during recovery in both non-acclimated and cold acclimated leaves.
Cluster 4only contained two genes encoding heat shock proteins of the HSP70 family (CPHSC 70–1 and BIP3).Both genes were upregulated throughout recovery in non-acclimated and cold acclimated leaves and showed a stable log2FC in comparison to the respective control with recovery time which was independent of the freezing temperature.
的ten genes incluster 5all showed strong up-regulation during recovery, which was, however, more pronounced in cold acclimated than in non-acclimated leaves. The three genes with the highest log2FC with values above five encoded O-glycosyl hydrolase family 17 protein (GH17), glucan endo-1,3-β-glucosidase 11 (GEG11) and GST TAU22 (GSTU22). Only at RAT, in particular in leaves from non-acclimated plants, some of the genes in cluster 5 showed no change in expression or even a mild (non-significant) down regulation.
Of the four genes incluster 6, two (UDP-glucose dehydrogenase 4(UDG4) andannexin 2(ANNAT2)) showed increased expression in leaves from cold acclimated plants at RAT and 1D-REC, in particular in leaves frozen to lower temperatures. In contrast,GSTU20was mainly down regulated, most strongly in non-acclimated leaves at 3D-REC and 6D-REC. Otherwise, the genes in cluster 6 did not show a coherent expression pattern in response to freezing or during recovery.
Finally,cluster 7contained genes that were mainly differentiated in their expression pattern directly after freezing (RAT). At RAT, most of these genes were down regulated in non-acclimated, but up regulated in cold acclimated leaves. At 1D-REC most of these genes were induced, in particular at lower freezing temperatures. This was especially pronounced for the three genes COR78,ERD10andCOR47.At the later recovery time points, the genes in this cluster tended to be less induced (3D-REC) or even strongly repressed (6D-REC) in their expression.
Discussion
Membrane repair and restored photosynthesis during recovery
Electrolyte leakage, as a measure of plasma membrane injury, was determined after leaves of non-acclimated and cold acclimated plants were frozen to different temperatures [31]。老鼠泄漏值增加而减少freezing temperatures, showing the direct temperature effect on the extent of leaf damage. Since RAT electrolyte leakage values were determined after over-night thawing, it cannot be excluded that some minor recovery had already taken place at that time. Freezing temperatures for the recovery experiments were chosen so that leaves either displayed recovery or irreversible injury. The latter was reached by freezing to temperatures below the LT50, which for non-acclimatedA. thalianaCol-0 has been established as close to − 6 °C and for cold acclimated plants as close to − 10 °C [28,32]。We found that freeze-thaw injury was irreversible for leaves of non-acclimated plants frozen to − 7 °C and of cold acclimated plants frozen to − 11 °C, corresponding to leakage values of about 45 and 67%, respectively. During the 6 day recovery period, non-acclimated as well as acclimated leaves that had been frozen to temperatures above the LT50随着时间的推移显示减少电解液泄漏。的largest decrease of electrolyte leakage occurred already at 1D-REC, suggesting that most recovery processes were activated rapidly after the initial freezing stress. This is in agreement with a substantial recovery from freezing damage in spinach leaves and oat crowns after 24 h [22,27]。
Recovery from freezing was additionally detected by measuring the quantum yield of PSII (Fv/Fm) using chlorophyll fluorescence imaging. In both non-acclimated and cold acclimated leaves, Fv/Fm decreased at the RAT time point with decreasing freezing temperatures as could be expected from previous publications [19,33]。Fv/Fm gradually recovered to values above the RAT value between 1D-REC and 3D-REC in all samples frozen to non-lethal temperatures. Decreasing the temperature by one degree (from − 6 °C to − 7 °C and from − 11 °C to − 12 °C for non-acclimated and cold acclimated leaves, respectively) not only prevented recovery of the photosynthetic efficiency completely, but actually resulted in further reduction in Fv/Fm over time. Presumably, the massive disruption of the thylakoid membrane system that takes place at these low temperatures [34] leads to further degradation of the photosynthetic machinery after thawing.
Fv/Fm did not reach the values obtained for UFC leaves even at 6D-REC except for non-acclimated samples when frozen to − 5 °C, suggesting that full repair of the photosynthetic machinery was not possible in Arabidopsis even after a relatively mild freezing stress, while in spinach leaves photosynthesis was fully recovered at 6D-REC [22]。Similarly, photosynthetic capacity recovered in Scots pine needles to prefrost levels within a few days [35], while full recovery of photosynthetic activity of Norway spruce needles after a frost event was only reached after 60 days [36]。
Moreover, the degree of recovery depended on the freezing temperature, indicating that the freezing temperature not only affected the initial damage, but also the extent of possible repair. Similarly, a rapid reactivation of photosynthesis after a 1 week warm spell was described for lingonberry (Vaccinium vitis-idaea) during different phases of winter whereas a prior freezing treatment retarded the recovery significantly [37]。
Respiration rate increased during recovery from freezing
We measured respiration rates at two different temperatures, namely the temperature at which leaves were kept for repair (4 °C) and in addition at 21 °C. As expected, respiration rates were three- to fourfold higher in UFC leaves at 21 °C compared to 4 °C. In leaves frozen to non-lethal temperatures, the initial respiration rate at RAT was generally higher in cold acclimated compared to non-acclimated leaves, independent of the measurement temperature. Cold-adapted plants have an increased number of mitochondria in their cells [38], resulting in a more active respiratory machinery after cold acclimation [39]。Decreasing temperatures additionally cause an accumulation of soluble sugars and starch [30]。Thus, an increased availability of substrate [40] could also contribute to an increase in respiration rates in cold acclimated leaves. Functionally, the increased respiration rates during recovery may be required to provide energy for biosynthetic and repair processes [41]。In addition, respiration may also be involved in ROS scavenging. Two terminal oxidases, cytochrome C oxidase (COX) and alternative oxidase (AOX) are involved in the respiratory pathway. AOX as non-energy conserving terminal oxidase greatly reduces the ATP yield during respiration because no proton-pumping across membranes is required due to bypassing of proton-pumping complex III and IV [42]。的AOXgene is upregulated during cold stress [43] which might lead to a reciprocal reduction of the COX pathway. Furthermore, silencing ofAOXinArabidopsiscauses accumulation of ROS [44]。Hence, it has been suggested that AOX acts as a ROS scavenger, further supported by an activation of the AOX respiratory pathway during recovery from injury [44]。
的recovery of non-acclimated samples was performed at a temperature of 4 °C, so the increase of the respiration rate in UFCs and samples frozen to − 6 °C and − 7 °C could also, at least partially, reflect the adaptation of the respiratory machinery to cold conditions in addition to the recovery process. Further, our data show that respiratory activity was still detectable in non-acclimated leaves frozen to − 8 °C at 6D-REC, when Fv/Fm was no longer detectable. This indicates that respiration is more robust under freezing conditions than photosynthesis, which may be functionally significant to ensure effective repair of partially damaged cells. Differential tolerance by various cellular processes to freeze-thaw treatment has previously been reported in potato leaves where photosynthesis was found to be much more sensitive than respiration [45]。
Sugars and proline may serve as energy and nitrogen sources during recovery
的initial sugar and proline content in unfrozen Arabidopsis control leaves was around ten times higher in acclimated than non-acclimated leaves, as reported previously [28,46]。Increases in sucrose, raffinose, and other cryoprotectants occur in parallel to a starch breakdown at the beginning of cold acclimation and transcriptional activation of genes encoding glycolytic enzymes supports the increasing demand for carbon skeletons for cryoprotectants [30]。
Soluble sugars and some amino acids such as proline stabilize both membranes and proteins during freezing (e.g. [47,48,49]). Moreover, both sugars [50,51] and proline [52,53] can act as ROS scavengers and may thereby also contribute to plant freezing tolerance. Obviously, the high amounts of sugars and proline that are accumulated during cold acclimation could also serve as carbon and nitrogen sources for repair processes after a freeze-thaw cycle, and the sugars could additionally provide the energy for repair through respiration, as discussed above.
UFC leaves from non-acclimated plants showed a continuous increase in the content of all four measured sugars over time. Since the leaves were incubated at 4 °C in the dark overnight and then at 4 °C with a 16 h day length during 6 days, we assume that this is due to a cold acclimation response. This conclusion is supported by an upregulation in the expression of the known cold-induced genesGolS3,COR6.6,COR15A,COR78andCOR47in these samples with expression values higher or similar to RAT (0 day control) values from cold acclimated plants (Additional file3:表S3)。有趣的是,脯氨酸没有significantly accumulate in non-acclimated leaves over 6 days under the same conditions. A delay in proline compared to sugar accumulation of approximately 1 day has been reported previously at 1 °C [54]。的longer delay observed in our experiments may be due to the fact that we investigated detached leaves while the earlier study was conducted with whole plants.
After freezing to sub-lethal temperatures, non-acclimated leaves showed very little change in sugar content during recovery, which may be due to increased sugar consumption by respiration, but may in addition also be related to damage to the photosynthetic machinery and reduced gluconeogenesis. The reduced sugar content in leaves frozen to the lowest temperature, where no repair was observed, was most likely related to a progressive breakdown of sub-cellular compartmentation under these conditions [55]。
In cold acclimated leaves sugars, except for glucose, were mostly decreased during recovery, probably caused by a higher demand for carbohydrates for respiration. Similarly, sucrose and fructose were strongly decreased after a 14 day recovery period after freezing in overwintering crown tissue ofAvena sativa[27]。
大多数从non-acclimat frozen-thawed叶子ed plants displayed a decrease in proline content during the first 3 days of recovery, while it increased again 6D-REC. A similar pattern was found in some of the cold acclimated samples, but with an increase in proline content already by 3D-REC. Proline may have been degraded during the early recovery phase to serve as nitrogen source for the repair of freezing injury. Once repair was largely finished, the demand for proline may have been reduced and cold-induced synthesis predominated again, leading to an increase in proline content. In cold acclimated leaves, more proline was available at the start of the repair process and therefore net proline accumulation was observed earlier despite its degradation. In overwintering, frozen-thawed crown tissue ofAvena sativa, proline, 5-oxoproline and arginine content increased over 14 days and this increase was significantly correlated with recovery [27]。At the lowest freezing temperatures, however, proline content slightly, but consistently decreased over time, in agreement with a lack of repair activity.
Changes in gene expression after freezing and during recovery
Immediately after freezing and during post-thaw recovery many changes in transcript abundance were observed, depending in a complex manner on the gene, the duration of recovery, the freezing temperature and the cold acclimation treatment. Cold-responsive genes (COR6.6,COR15A, GOLS3,COR78,COR47) were immediately downregulated after freezing in non-acclimated leaves, and were either unchanged or upregulated in cold acclimated leaves. In both cases they showed an upregulation or no change at 1D-REC and a consistent downregulation at 3D-REC and 6D-REC, indicating a decreasing role of cold-responsive genes during recovery. Together with the reduction in osmolyte content this suggests a general rerouting of metabolism from maintaining freezing tolerance towards repair.
的three investigated aquaporin genes (PIP2A,PIP2E,TIP2) showed reduced transcript abundance directly after freezing and throughout the recovery period. The expression of aquaporin genesPIP2.1andTIPwas also downregulated in spinach leaves injured by mild freezing, butPIP2.1expression was partially restored at 3D-REC and 6D-REC, whileTIPexpression increased 1.5-fold over the control level at 6D-REC [22]。
Cell wall remodeling is an important process during recovery from freezing [16] and we have investigated the expression changes of eight genes encoding enzymes involved in this process. FourUGDgenes were induced over the recovery period in both non-acclimated and cold acclimated leaves. The corresponding enzymes are involved in the biosynthesis of UDP-glucuronic acid for the synthesis of pectins and hemicelluloses [56]。A distinct role of the different UGD proteins in carbon partitioning between cell wall synthesis and sucrose synthesis was suggested [56]。An overexpression of anUGDgene fromLarix gmeliniiin Arabidopsis resulted in increased levels of sugars and hemicellulose combined with enhanced growth and freezing tolerance [57]。In addition,AXS2, which encodes an enzyme that converts UDP-D-glucuronate to a mixture of UDP-D-apiose and UDP-D-xylose, was also induced during recovery. D-apiose is found in rhamnogalacturonan II, apiogalacturonan, and several apioglycosides. An UDP-apiose/xylose synthase was among the most highly induced proteins during recovery from freezing in onion [13]。
GH17andGEG11with induced expression during the whole recovery phase encode proteins involved in the hydrolysis of β-1,3-glucoside linkages [58]。An increase of β-glucanase activity in the cold has also been described in winter rye [59]。In addition to their enzymatic function that relates them to cell wall remodeling, β-1,3-glucanases also have ice binding and ice recrystallization inhibition activity [59] and are able to directly stabilize plant membranes during freezing [60]。
的massive upregulation of twoGSTgenes (GSTU19andGSTU22) during recovery may be taken as evidence for the need of additional ROS scavenging under these conditions. Indeed, it has been shown previously that in spinach leaves both superoxide and H2O2are accumulated directly after thawing from a sub-lethal freezing temperature and that these ROS are rapidly reduced during recovery [22]。At the same time the activity of the antioxidant enzymes catalase, ascorbate peroxidase and superoxide dismutase is increased. Antioxidant enzymes including several GSTs increased in onion during repair of freeze-thaw-injury [13]。Enzymes with antioxidative function or their transcripts also showed higher abundance during recovery from drought stress [61]。
GSTs are involved in cellular protection against oxidative stress and in particular in regulation of the H2O2balance [62]。的y reduce oxidative stress by catalyzing the formation of a disulfide bridge between two glutathione molecules, thus forming glutathione disulfide. The electrons released in this reaction are then available for the reduction of radicals [62]。GSTs can in addition bind to a variety of exogenous and endogenous ligands which might be damaging to the cell [63]。GSTU19, for instance, catalyzes the glutathionylation of 12-oxophytodienoate (OPDA), a precursor of jasmonic acid. The resulting OPDA-GSH conjugate is then stored in the vacuole [64]。GSTs can also function as carrier proteins for the phytohormones auxin and cytokinin and bind fragments of chlorophyll [65]。Whether any of these functions that are not directly related to ROS scavenging play a role in repair remains to be investigated.
Freezing can cause protein aggregation induced by cell contraction and concentration of the cytoplasm [13] and soluble enzymes may be inactivated during an in vivo freeze-thaw cycle [20]。During repair, such proteins need to be either proteolytically removed or renatured by chaperones. In agreement with this proposition, several HSP genes and proteins, respectively, were induced during recovery in onion [13] and spinach [22]。HSPs are involved in proteostasis, the maintenance of the cellular proteome. We determined the expression of five genes encoding HSP70 family proteins during the recovery process. All five genes (BIP1, BIP2,BIP3,CPHSC70–1andCPHSC70–2) were highly up regulated during recovery. BIP proteins are HSP70 proteins of the ER and are able to bind client proteins to prevent their aggregation [66]。BIPs function as chaperones in processes such as protein folding, protein translocation and quality control [67]。的y also act as master regulators of Arabidopsis stress responses [68] and play a putative role in the assembly of multimeric protein complexes in the ER [66]。
Two otherHSP70genes (CPHSC70–1andCPHSC70–2) were also upregulated throughout recovery. They are nuclear encoded and transported into chloroplasts [69]。A knock-out mutant ofCPHSC70–1is retarded in growth and has abnormal leaves, in contrast to aCPHSC70–2mutant that shows no phenotypic effect under normal growth conditions [69]。However, double mutants of both genes are lethal. Artificial microRNA approaches showed that these genes are essential for a normal plastid structure [70]。
两个基因编码14-3-3蛋白质regulated during recovery, the general regulatory factorsGRF5andGRF7.This is in agreement with proteomic data in onion, showing increased abundance of two 14–3-3 proteins during recovery [13]。Both Arabidopsis proteins are phosphorylated by cold-responsive protein kinase 1 (CRPK1), inducing their translocation to the nucleus where they interact with CBF transcription factors and promote their degradation [71]。Knockout ofGRF7结果在增强和超表达减少freezing tolerance. GRF5 is in addition involved in cytoskeleton organization by activating the microtubule-associated protein Endosperm defective 1 (EDE1), which is essential for cell division and microtubule organization during early stages of mitosis [72]。的ir role as negative regulators of the CBF regulon is in agreement with our hypothesis that plants actively shift their metabolism from maintaining or inducing their freezing tolerance in favor of repair processes.
Conclusions
In the present paper we have addressed research questions central to understanding repair processes in leaves after freezing, highlighting its dependence on the severity of damage, as well as its metabolic and transcriptional basis. Leaves of non-acclimated and cold acclimated Arabidopsis plants showed a fast and continuous recovery, measured as membrane repair accompanied by a restored photosynthesis, only after freezing to sub-lethal but not to lethal temperatures. Increased respiration rates and unchanged or declining levels of compatible solutes, such as sugars and proline, were suggested to act as energy, carbon and nitrogen sources for the repair process. Higher expression of genes encoding proteins important for cell wall remodeling, ROS scavenging and maintenance of the cellular proteome during recovery occurred in parallel with a down regulation of cold-responsive genes, especially in cold acclimated leaves. Transcriptional regulation, e.g. by 14–3-3 proteins, was suggested to be involved in post-thaw recovery. This work contributes to a deeper understanding of recovery processes after sub-lethal freeze-thaw injury, which gains increasing importance due to global climate change and highly fluctuating temperatures that have an increasing effect on plant survival.
Methods
Plant material
Arabidopsis thalianaaccession Columbia-0 (Col-0) originally ordered from NASC (Nottingham Arabidopsis Stock Center, United Kingdom) and propagated for several years at the MPI-MP (Potsdam, Germany) was used in all experiments. After sowing, plants were grown on soil in a climate chamber at 20 °C day-time temperature and 6 °C night-time temperature in a 12 h day light-dark cycle with a light intensity of 180 μE m− 2 s− 1and humidity of 70% (day) and 80% (night) for 1 week. The plants were then grown at short day conditions (8 h day length, 180 μE m− 2 s− 1, humidity of 60/75% day/night) with temperatures of 20 °C and 16 °C (day/night) for 3 weeks. Then plants were transferred to long day conditions (16 h light) at 20 °C day and 18 °C night temperature and light intensity of 200 μE m− 2 s− 12周。这些增长6周后,non-acclimated plants were used in experiments. For cold acclimation, plants were kept for an additional week in a growth chamber at 4 °C and a 16 h day length (90 μE m− 2 s− 1light intensity, 70–80% humidity) [28]。
For recovery, detached leaves in glass tubes were transferred back after freezing and thawing to the growth chamber used for cold acclimation for 1, 3 or 6 days. Leaves in tubes were kept out of direct light at 15 μE m− 2 s− 1light intensity to prevent damage caused by photooxidative processes. Measurements were performed on the leaves right after thawing (RAT) and after 1, 3 or 6 days of recovery (REC) at 8:30 am (2 ½ h after lights on). In addition, samples were taken for proline, sugar and transcript analysis and were stored at − 80 °C.
Freezing and electrolyte leakage
Freezing experiments were performed according to the method previously described [31]。Two to three leaves, collected from individual plants, were transferred to glass tubes containing 300 μl of water. Control tubes were kept on ice throughout the experiment (unfrozen control – UFC, 1D-UFC, 3D-UFC, 6D-UFC), whereas all other tubes were moved to a programmable silicon oil bath CC130 (Huber, Offenburg, Germany) at a temperature of − 1 °C. After half an hour, ice was added to initiate ice crystallization in the leaves. After an additional 30 min, the oil bath was set to a cooling rate of 4 °C per h. Freezing temperatures above or below the LT50, the temperature where 50% of electrolytes leak out of the cell, of non-acclimated (LT50NA) (− 3.0 °C, − 3.5 °C, − 4.5 °C, − 5 °C or − 7 °C) or cold acclimated plants (LT50ACC) (− 8.0 °C, − 8.5 °C or − 11 °C) were chosen according to previously published results (LT50NA: -5.34 °C NA, LT50ACC: -9.68 °C) [28] (Table1). Samples were removed from the bath at appropriate temperatures and left on ice to thaw overnight. Leaves were covered with 7 ml of water and tubes were gently shaken for 24 h at 4 °C before measuring electrolytes at RAT conditions. For samples after 1D-REC, 3D-REC or 6D-REC at 4 °C, water was added at the respective day and electrolyte leakage was measured as above. A detailed sampling scheme is shown in Additional file5: Figure S2.
For electrolyte leakage measurements, 4.5 ml deionized water was mixed with 1 ml of the water used to shake the leaves in and conductivity was measured. Afterwards tubes containing the plant material and the remaining water were boiled for 30 min and left to cool to room temperature before a second measurement was carried out to obtain the 100% electrolyte leakage value for each sample. The ratio between the two values was calculated and control values were subtracted to obtain the final electrolyte leakage values. For the characterization of recovery processes one experiment was performed with four biological replicates derived from different sets of plants for each freezing temperature and condition.
For all other analyses leaf samples were collected after freezing to lower temperatures than for the electrolyte leakage experiments, since the 24 h-incubation in water, which leads to additional damage to the leaf tissue, was not necessary [19]。For chlorophyll fluorescence imaging leaves were frozen to − 5 °C, − 6 °C and − 7 °C to elicit non-lethal and − 8 °C to elicit lethal damage to non-acclimated plants, or − 9 °C, − 10 °C and − 11 °C (non-lethal) and − 12 °C and − 13 °C (lethal) using cold acclimated plants (Table1). Since respiration measurements were done at two different temperatures (4 °C and 21 °C) only a limited number of samples could be processed and only samples frozen to − 6 °C and − 7 °C for non-lethal and − 8 °C for lethal damage using non-acclimated plants, or − 8 °C and − 9 °C for non-lethal and − 12 °C for lethal damage using cold acclimated plants were measured. For sugar and proline analysis, additional samples were analyzed that have been frozen to − 4.5 °C for non-acclimated plants or − 8 °C and − 8.5 °C for cold acclimated plants to generate an overlap with freezing temperatures used for electrolyte leakage measurements. Samples were always taken 1 day after freezing (RAT) and 1D-REC, 3D-REC and 6D-REC.
Chlorophyll fluorescence imaging
DetachedArabidopsisleaves were frozen and thawed as described above. Samples were dark adapted for at least 30 min before measuring chlorophyll fluorescence using an IPAM (imaging pulsed-amplitude modulated) fluorometer (Walz, Germany) [31]。的false color images of two to three leaves from five replicates each per freezing temperature and condition were taken (n = 10–15) per experiment and data processed using image processing software provided with the instrument (ImagingWinGigE_246r, Walz). The whole leaf area was integrated to calculate a mean Fv/Fm reflecting the potential quantum use efficiency of photosystem II [73]。Three independent experiments were performed including samples frozen to − 6 °C, − 8 °C, − 9 °C, − 10 °C, two experiments for samples frozen to − 13 °C and one experiment including samples frozen to − 5 °C, − 7 °C, − 11 °C and − 12 °C.
Respiration
Respiration measurements were performed as described previously [74]。叶子暗适应了30分钟,然后勒af slices were cut from two leaves per replicate in dim light and placed into 1.5 ml vials (Supelco Analytical, Bellefonte, CA) filled with 25 mM imidazole buffer (pH 6.5). Air bubbles were removed using an ultra-sonic water bath Sonorex RK100 (Bandelin, Berlin, Germany). Vials were topped up with buffer and closed with screw caps containing a silicone inlet (Chromacol, Welwyn Garden City, England) without trapping air bubbles. Vials were incubated in darkness at either 21 °C or 4 °C. Oxygen consumption was measured in the dark at three consecutive time points with a Microx TX2 system and a needle mounted optical oxygen microsensor (NTH Pst 1-LS-TS-NS 40 × 0, PreSens, Regensburg, Germany) using the program TX2 OxyView V4.2 (PreSens). The sensor was calibrated against ambient air (100% oxygen saturation) and a 1% (w/v) sodium sulfite solution (0% oxygen saturation). After measurement, leaf slices were removed from the vials, shortly dried on paper and weighed. Respiration rates were calculated as nmol O2min− 1 mg− 1FW. Experiments were performed with five replicates per freezing temperature and recovery condition.
Sugar and proline measurements
Sugars and proline were quantified from detached leaves after the different treatments as previously described [28]。Samples were frozen in liquid nitrogen and homogenized using a ball mill ‘Retsch MM 200’ (Retsch, Haan, Germany). Soluble sugars were extracted with 80% ethanol from 50 mg of frozen leaf material, desalted and quantified by high performance anion exchange chromatography using a CarboPac PA-100 column on an ICS3000 chromatography system (Dionex, Sunnyvale, CA, USA). Proline was quantified photometrically from the same ethanol extracts using the acidic ninhydrin method [28,75]。Samples from one experiment were measured with five replicates per freezing temperature at RAT and 1D-REC, 3D-REC and 6D-REC except for the non-acclimated UFC, − 6 °C and − 8 °C with three experiments measured.
qRT-PCR analysis of gene expression
Total RNA was extracted from leaf material pooled from five replicate samples per freezing temperature and recovery condition from up to four biological experiments. For the control samples and − 9 °C for cold acclimated samplesn = 4, for − 6 °C, − 8 °C, − 13 °C and for the non-acclimated controln = 3. For − 5 °C and − 11 °Cn = 2 and for − 7 °C, − 9 °C, − 12 °Cn = 1. RNA extraction, DNAse treatment and RNA quality control using forward and reverse intron specific primers (MAF5 primer, AT5G65080) was performed according to a previously described method [76]。First strand cDNA synthesis and cDNA quality controls using forward and reverse 3’GADPH and 5’GADPH primers (AT1G13440) and quantitative PCR measurements were performed as published [28]。Reactions with 2.5 μl 2 × SYBR Green Master Mix (Fast Power SYBR Green; Applied Biosystems, Darmstadt, Germany), 0.5 μl cDNA (diluted 5-fold) and 2 μl of 0.5 μM primers were pipetted using an Evolution P3 pipetting robot (PerkinElmer, Zaventem, Belgium) and measured using an ABI PRISM 7900 HT 384-well plate Sequence Detection System (Applied Biosystems).
Genes of interest for the expression analysis were chosen from a proteomic analysis of onion scales comparing UFC, freeze-thaw injured and post-thaw recovered tissues [13]。Orthologs of potential recovery related proteins in onion were identified in Arabidopsis (Additional file1: Table S1) and respective gene sequences were selected together with some related genes for primer design. Primers were either designed in Primer3 (http://primer3.wi.mit.edu/) [77,78] or taken from the literature. The specifications of the designed primers were as follows: primer length 20–24 bases, amplicon size 60–150 bp, primer melting temperature 64 ± 3 °C, amplicon melting temperature 75–95 °C, G/C content 45–55%, maximum repetition of a nucleotide 3 and a G/C clamp of 1. Further, primers that hybridize close to the 3′ end of the gene were preferably chosen. Sequences of all primers can be found in Additional file2: Table S2. Ct values for the genes of interest were normalized by subtracting the mean Ct of the three reference genes,Actin2,EXPRSandPDF2[28], and averages of all biological replicates were determined. Relative expression changes were calculated as log2fold-change compared to the respective control and were visualized in heat maps generated using Pheatmap in R [79]。Hierarchical cluster analysis was performed using Pearson correlation with average linkage. The significance of differences between the conditions was calculated using an unpaired two-sided t-test in Excel.
Further data analysis methods
Principle Components Analysis (PCA) was performed using ∆Ct values, which were normalized to the median of all samples for each gene and taken to the log10. Probabilistic PCA was performed using thepcaMethodspackage in R [80]。
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files or are available from the corresponding author on request.
Abbreviations
- ACC:
-
Cold acclimated
- AOX:
-
Alternative oxidase
- COR:
-
Cold responsive genes
- COX:
-
Cytochrome C oxidase
- D-REC:
-
Day after recovery
- FTST:
-
Freeze-thaw stress tolerance
- FW:
-
Fresh weight
- GSH:
-
Glutathione
- HSP:
-
Heat shock protein
- LT50:
-
Leakage temperature at which 50% of damage occurs
- NA:
-
Non-acclimated
- PC:
-
Principal component
- PCA:
-
Principal component analysis
- RAT:
-
Right after thawing
- ROS:
-
Reactive oxygen species
- UFC:
-
Unfrozen control
References
- 1.
Levitt J. Responses of plants to environmental stresses. Volume I: Chilling, freezing, and high temperature stresses. In: Kozlowski TT, editor. Physiological ecology. New York: Academic Press; 1980. p. 497.
- 2.
Hincha DK, Espinoza C, Zuther E. Transcriptomic and metabolomic approaches to the analysis of plant freezing tolerance and cold acclimation. In: Improving crop resistance to abiotic stress. Weinheim: Wiley-VCH Verlag GmbH & Co. KG; 2012. p. 255–87.
- 3.
Vyse K, Pagter M, Zuther E, Hincha DK. Deacclimation after cold acclimation - a crucial, but widely neglected part of plant winter survival. J Exp Bot. 2019;70(18):4595–604.
- 4.
Wiest SC, Steponkus PL. Freeze-thaw injury to isolated spinach protoplasts and its simulation at above freezing temperatures. Plant Physiol. 1978;62(5):699–705.
- 5.
Uemura M, Tominaga Y, Nakagawara C, Shigematsu S, Minami A, Kawamura Y. Responses of the plasma membrane to low temperatures. Physiol Plant. 2006;126(1):81–9.
- 6.
Warmund MR, Guinan P, Fernandez G. Temperatures and cold damage to small fruit crops across the Eastern United States associated with the April 2007 freeze. Hortic Sci. 2008;43(6):1643–7.
- 7.
Gu L, Hanson PJ, Post WM, Kaiser DP, Yang B, Nemani R, Pallardy SG, Meyers T. The 2007 Eastern US spring freeze: increased cold damage in a warming world? BioScience. 2008;58(3):253–62.
- 8.
Augspurger CK. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: spring damage risk is increasing. Ecology. 2013;94(1):41–50.
- 9.
Unterberger C, Brunner L, Nabernegg S, Steininger KW, Steiner AK, Stabentheiner E, Monschein S, Truhetz H. Spring frost risk for regional apple production under a warmer climate. PLoS One. 2018;13:7.
- 10.
Richardson AD, Hufkens K, Milliman T, Aubrecht DM, Furze ME, Seyednasrollah B, Krassovski MB, Latimer JM, Nettles WR, Heiderman RR, et al. Ecosystem warming extends vegetation activity but heightens vulnerability to cold temperatures. Nature. 2018;560(7718):368–71.
- 11.
Steponkus PL. Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol. 1984;35(1):543–84.
- 12.
Palta JP, Levitt J, Stadelmann EJ. Freezing injury in onion bulb cells: II. Post-thawing injury or recovery. Plant Physiol. 1977;60(3):398–401.
- 13.
Chen K, Renaut J, Sergeant K, Wei HUI, Arora R. Proteomic changes associated with freeze-thaw injury and post-thaw recovery in onion (Allium cepa L.) scales. Plant Cell Environ. 2013;36(4):892–905.
- 14.
Arora R, Palta JP. Perturbation of membrane calcium as a molecular mechanism of freezing injury. In: Cherry JH, editor. Environmental stress in plants. Berlin-Heidelberg: Springer; 1989. p. 281–90.
- 15.
我喜欢LV, Wisniewski, Trischuk RG。的模式freezing in plants: the influence of species, environment and experiential procedures. In: Gusta LV, Wisniewski ME, Tanino KK, editors. Plant cold hardiness: from the laboratory to the field. Wallingford: CABI; 2009. p. 214–25.
- 16.
Arora R. Mechanism of freeze-thaw injury and recovery: a cool retrospective and warming up to new ideas. Plant Sci. 2018;270:301–13.
- 17.
Takahashi D, Bin L, Nakayama T, Kawamura Y, Uemura M. Plant plasma membrane proteomics for improving cold tolerance. Front Plant Sci. 2013;4:1–5.
- 18.
Hincha D, Sieg F, Bakaltcheva I, Köth H, Schmitt J. Freeze-thaw damage to thylakoid membranes: specific protection by sugars and proteins. In: Steponkus P, editor. Advances in low-temperature biology. London: JAI Press; 1996. p. 141–83.
- 19.
Ehlert B, Hincha DK. Chlorophyll fluorescence imaging accurately quantifies freezing damage and cold acclimation responses in Arabidopsis leaves. Plant Methods. 2008;4(1):12.
- 20.
Thalhammer A, Bryant G, Sulpice R, Hincha DK. Disordered cold regulated15 proteins protect chloroplast membranes during freezing through binding and folding, but do not stabilize chloroplast enzymes in vivo. Plant Physiol. 2014;166(1):190–201.
- 21.
Arora R, Palta JP. A loss in the plasma membrane ATPase activity and its recovery coincides with incipient freeze-thaw injury and postthaw recovery in onion bulb scale tissue. Plant Physiol. 1991;95(3):846–52.
- 22.
Chen K, Arora R. Understanding the cellular mechanism of recovery from freeze–thaw injury in spinach: possible role of aquaporins, heat shock proteins, dehydrin and antioxidant system. Physiol Plant. 2014;150(3):374–87.
- 23.
Zuther E, Juszczak I, Lee YP, Baier M, Hincha DK. Time-dependent deacclimation after cold acclimation inArabidopsis thalianaaccessions. Sci Rep. 2015;5:12199.
- 24.
Pagter M, Alpers J, Erban A, Kopka J, Zuther E, Hincha DK. Rapid transcriptional and metabolic regulation of the deacclimation process in cold acclimatedArabidopsis thaliana.BMC Genomics. 2017;18(1):731.
- 25.
Carpenter JF, Crowe JH. The mechanism of cryoprotection of proteins by solutes. Cryobiology. 1988;25(3):244–55.
- 26.
Tarkowski ŁP, van den Ende W. Cold tolerance triggered by soluble sugars: a multifaceted countermeasure. Front Plant Sci. 2015;6:203.
- 27.
Henson CA, Duke SH, Livingston DP 3rd. Metabolic changes inAvena sativacrowns recovering from freezing. PLoS One. 2014;9:3.
- 28.
Zuther E, Schulz E, Childs LH, Hincha DK. Clinal variation in the non-acclimated and cold-acclimated freezing tolerance ofArabidopsis thalianaaccessions. Plant Cell Environ. 2012;35(10):1860–78.
- 29.
Atkin OK, Tjoelker MG. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003;8(7):343–51.
- 30.
Guy C, Kaplan F, Kopka J, Selbig J, Hincha DK. Metabolomics of temperature stress. Physiol Plant. 2008;132(2):220–35.
- 31.
Thalhammer A, Hincha DK, Zuther E. Measuring freezing tolerance: electrolyte leakage and chlorophyll fluorescence assays. In: Hincha DK, Zuther E, editors. Plant cold acclimation: methods and protocols. New York: Springer; 2014. p. 15–24.
- 32.
Klotke J, Kopka J, Gatzke N, Heyer AG. Impact of soluble sugar concentrations on the acquisition of freezing tolerance in accessions ofArabidopsis thalianawith contrasting cold adaptation – evidence for a role of raffinose in cold acclimation. Plant Cell Environ. 2004;27(11):1395–404.
- 33.
Knaupp M, Mishra KB, Nedbal L, Heyer AG. Evidence for a role of raffinose in stabilizing photosystem II during freeze–thaw cycles. Planta. 2011;234(3):477–86.
- 34.
Hincha DK, Höfner R, Schwab KB, Heber U, Schmitt JM. Membrane rupture is the common cause of damage to chloroplast membranes in leaves injured by freezing or excessive wilting. Plant Physiol. 1987;83(2):251–3.
- 35.
Ensminger我,施密特L,劳埃德·j .土壤温度and intermittent frost modulate the rate of recovery of photosynthesis in Scots pine under simulated spring conditions. New Phytol. 2008;177(2):428–42.
- 36.
Wallin G, Hall M, Slaney M, Räntfors M, Medhurst J, Linder S. Spring photosynthetic recovery of boreal Norway spruce under conditions of elevated [CO2] and air temperature. Tree Physiol. 2013;33(11):1177–91.
- 37.
Saarinen T, Lundell R, Hänninen H. Recovery of photosynthetic capacity inVaccinium vitis-idaeaduring mild spells in winter. Plant Ecol. 2011;212(9):1429–40.
- 38.
Miroslavov EA, Kravkina IM. Comparative analysis of chloroplasts and mitochondria in leaf chlorenchyma from mountain plants grown at different altitudes. Ann Bot. 1991;68(3):195–200.
- 39.
Körner C. Plant adaptation to cold climates. F1000Research. 2016;5:2769.
- 40.
Paul MJ, Driscoll SP, Lawlor DW. Sink-regulation of photosynthesis in relation to temperature in sunflower and rape. J Exp Bot. 1992;43(2):147–53.
- 41.
Talts P, Pärnik T, Gardeström P, Keerberg O. Respiratory acclimation inArabidopsis thalianaleaves at low temperature. J Plant Physiol. 2004;161(5):573–9.
- 42.
Vanlerberghe GC. Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int J Mol Sci. 2013;14(4):6805–47.
- 43.
Matos AR, Hourton-Cabassa C, Ciçek D, Rezé N, Arrabaça JD, Zachowski A, Moreau F. Alternative oxidase involvement in cold stress response ofArabidopsis thaliana fad2andFAD3+cell suspensions altered in membrane lipid composition. Plant Cell Physiol. 2007;48(6):856–65.
- 44.
Umbach AL, Fiorani F, Siedow JN. Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol. 2005;139(4):1806–20.
- 45.
Steffen KL, Arora R, Palta JP. Relative sensitivity of photosynthesis and respiration to freeze-thaw stress in herbaceous species : importance of realistic freeze-thaw protocols. Plant Physiol. 1989;89(4):1372–9.
- 46.
Lee YP, Babakov A, de Boer B, Zuther E, Hincha DK. Comparison of freezing tolerance, compatible solutes and polyamines in geographically diverse collections of的llungiella sp.andArabidopsis thalianaaccessions. BMC Plant Biol. 2012;12:131.
- 47.
Santarius KA. Freezing of isolated thylakoid membranes in complex media. VIII. Differential cryoprotection by sucrose, proline and glycerol. Physiol Plant. 1992;84(1):87–93.
- 48.
Anchordoguy TJ, Rudolph AS, Carpenter JF, Crowe JH. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology. 1987;24(4):324–31.
- 49.
Crowe JH, Carpenter JF, Crowe LM, Anchordoguy TJ. Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology. 1990;27(3):219–31.
- 50.
Gechev TS, van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays. 2006;28(11):1091–101.
- 51.
Stoyanova S, Geuns J, Hideg É, van den Ende W. The food additives inulin and stevioside counteract oxidative stress. Int J Food Sci Nutr. 2011;62(3):207–14.
- 52.
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments: a review. Plant Signal Behav. 2012;7(11):1456–66.
- 53.
Szabados L, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15(2):89–97.
- 54.
Wanner LA, Junttila O. Cold-induced freezing tolerance in Arabidopsis. Plant Physiol. 1999;120:391–400.
- 55.
Hincha D, Müller M, Hillmann T, Schmitt J. Osmotic stress causes mechanical freeze-thaw damage to thylakoids in vitro and in vivo. In: Cherry J, editor. Environmental stress in plants. Berlin: Springer; 1989. p. 303–15.
- 56.
Klinghammer M, Tenhaken R. Genome-wide analysis of the UDP-glucose dehydrogenase gene family in Arabidopsis, a key enzyme for matrix polysaccharides in cell walls. J Exp Bot. 2007;58(13):3609–21.
- 57.
Li NN, Chen L, Li XH, Li Q, Zhang WB, Takechi K, Takano H, Lin XF. Overexpression of UDP-glucose dehydrogenase fromLarix gmeliniienhances growth and cold tolerance in transgenicArabidopsis thaliana.Biol Plant. 2017;61(1):95–105.
- 58.
Henrissat B, Callebaut I, Fabrega S, Lehn P, Mornon JP, Davies G. Conserved catalytic machinery and the prediction of a common fold for several families of glycosyl hydrolases. Proc Natl Acad Sci U S A. 1995;92(15):7090–4.
- 59.
Yaish MWF, Doxey AC, McConkey BJ, Moffatt BA, Griffith M. Cold-active winter rye glucanases with ice-binding capacity. Plant Physiol. 2006;141(4):1459–72.
- 60.
Hincha DK, Meins F Jr, Schmitt JM. β-1,3-glucanase is cryoprotective in vitro and is accumulated in leaves during cold acclimation. Plant Physiol. 1997;114(3):1077–83.
- 61.
Chaves MM, Oliveira MM. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot. 2004;55(407):2365–84.
- 62.
Dixon DP, Lapthorn爱德华r .植物谷胱甘肽transferases. Genome Biol. 2002;3(3):Reviews3004.
- 63.
Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360(Pt 1):1–16.
- 64.
Ohkama-Ohtsu N, Sasaki-Sekimoto Y, Oikawa A, Jikumaru Y, Shinoda S, Inoue E, Kamide Y, Yokoyama T, Hirai MY, Shirasu K, et al. 12-oxo-phytodienoic acid-glutathione conjugate is transported into the vacuole in Arabidopsis. Plant Cell Physiol. 2011;52(1):205–9.
- 65.
Marrs KA. The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:127–58.
- 66.
Cho Y, Kanehara K. Endoplasmic reticulum stress response in Arabidopsis roots. Front Plant Sci. 2017;8:144.
- 67.
Maruyama D, Endo T, Nishikawa S-I. BiP3 supports the early stages of female gametogenesis in the absence of BiP1 and BiP2 inArabidopsis thaliana.Plant Signal Behav. 2015;10(7):e1035853.
- 68.
Srivastava R, Deng Y, Shah S, Rao AG, Howell SH. BINDING PROTEIN is a master regulator of the endoplasmic reticulum stress sensor/transducer bZIP28 in Arabidopsis. Plant Cell. 2013;25(4):1416–29.
- 69.
Su PH, Li HM. Arabidopsis stromal 70-kD heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol. 2008;146(3):1231–41.
- 70.
Latijnhouwers M, Xu XM, Moller SG. Arabidopsis stromal 70-kDa heat shock proteins are essential for chloroplast development. Planta. 2010;232(3):567–78.
- 71.
Liu Z, Jia Y, Ding Y, Shi Y, Li Z, Guo Y, Gong Z, Yang S. Plasma membrane CRPK1-mediated phosphorylation of 14–3-3 proteins induces their nuclear import to fine-tune CBF signaling during cold response. Mol Cell. 2017;66(1):117–128.e115.
- 72.
Pignocchi C, Doonan JH. Interaction of a 14-3-3 protein with the plant microtubule-associated protein EDE1. Ann Bot. 2011;107(7):1103–9.
- 73.
Lichtenthaler HK, Miehé JA. Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci. 1997;2(8):316–20.
- 74.
Glaubitz U, Li X, Köhl KI, van Dongen JT, Hincha DK, Zuther E. Differential physiological responses of different rice (Oryza sativa) cultivars to elevated night temperature during vegetative growth. Funct Plant Biol. 2014;41(4):437–48.
- 75.
Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–7.
- 76.
Sprenger H, Kurowsky C, Horn R, Erban A, Seddig S, Rudack K, Fischer A, Walther D, Zuther E, Kohl K, et al. The drought response of potato reference cultivars with contrasting tolerance. Plant Cell Environ. 2016;39(11):2370–89.
- 77.
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3 - new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115.
- 78.
Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23(10):1289–91.
- 79.
Kolde R. pheatmap: Pretty heatmaps [Software]; 2015.
- 80.
Stacklies W, Redestig H, Scholz M, Walther D. Selbig J: pcaMethods--a bioconductor package providing PCA methods for incomplete data. Bioinformatics. 2007;23(9):1164–7.
Acknowledgements
We thank Astrid Basner and Jessica Alpers for their excellent technical assistance with the sugar and qRT-PCR measurements and Marc Aurel Schöttler for help with the chlorophyll fluorescence measurements.
Funding
This work was supported by the German Research Foundation (DFG, Collaborative Research Center 973, Grant No. 190578797) through funds for project A3 to DKH and a travel grant to RA for his sabbatical assignment at MPI-MP from the Collaborative Research Center 973. The funders played no role in study design, in the decision to publish and in writing the manuscript.
Author information
Affiliations
Contributions
DKH, RA和EZ构思项目,KV,摩根大通,EZnd RA performed the experiments, KS provided the candidate genes from Arabidopsis, KV performed all other data analyses, KV, DKH and EZ, wrote the manuscript with contributions from all co-authors. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
的authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Orthologs of potential recovery related proteins identified by a proteomic study in onion [13] and identified in Arabidopsis by BLAST search.
Additional file 2: Table S2.
Sequences of the primers used for qRT-PCR analysis.
Additional file 3: Table S3.
Relative expression (2-∆Ct) of 41 genes in Arabidopsis leaves frozen to different temperatures right after thawing (RAT) or after recovery for 1 day (1D-REC), 3 days (3D-REC) or 6 days (6D-REC) at 4 °C for non-acclimated and cold acclimated plants.
Additional file 4: Figure S1.
Probabilistic Principal Component Analysis (PCA) using expression data of 41 genes measured by qRT-PCR in Arabidopsis leaves frozen to different temperatures right after thawing or after recovery for 1, 3 or 6 days at 4 °C for non-acclimated (NA) plants (red dots) and cold acclimated (ACC) plants (blue dots). The mean gene expression was divided by the median gene expression across all condition and the log10 was calculated.
Additional file 5: Figure S2.
Sampling scheme for the recovery of detached leaves from non-acclimated and cold acclimated plants after freezing. RAT – right after thawing, REC – recovery, UFC – unfrozen control, D - day.
Rights and permissions
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vyse, K., Penzlin, J., Sergeant, K.et al.Repair of sub-lethal freezing damage in leaves ofArabidopsis thaliana.BMC Plant Biol20,35 (2020). https://doi.org/10.1186/s12870-020-2247-3
Received:
Accepted:
Published:
Keywords
- Freezing damage
- Repair
- Arabidopsis
- Respiration
- Photosynthesis
- Gene expression